Introduction
Hydrogen is an environmentally friendly energy carrier that, unlike electricity, can be stored in large quantities. It can be converted into electricity in fuel cells, with only heat and water as by-products.
It is also compatible with combustion turbines and reciprocating engines to produce power with nearzero emission of pollutants. Therefore, hydrogen could play a major role in energy systems and serve all sectors of the economy, substituting for fossil fuels and helping mitigate global warming.
Nuclear energy, in addition to its application for producing electricity, can also be used to generate hydrogen for direct use by energy consumers. Generating hydrogen using nuclear energy has important potential advantages over other processes. For example, it requires no fossil fuels, results in lower greenhouse-gas emissions and other pollutants, and can lend itself to large-scale production. As a greenhouse-gas-free alternative, methods of using nuclear energy to produce hydrogen from water by electrolysis, thermochemical, and hybrid processes are being explored. This paper briefly describes these three different processes.
Initial Considerations Regarding Reactor Types and Processes
As long as it can provide electricity and process heat, any type of nuclear reactor can be used for the production of hydrogen. However, the reactor coolant and its maximum temperature are essential criteria for determining which reactor type is more appropriate for different production processes. Power size is also an important factor, as large reactors are more suitable for cogeneration of electricity and hydrogen production, whereas small sized plants are more suitable as single purpose plants (e.g. for hydrogen production only).
Specific considerations are more relevant for specific types of reactors. For instance, the cost of hydrogen generation would be less attractive for a small sized plant to be used for hydrogen production only, as could be the case when using light water reactors for hydrogen production. However, it is possible to make this process more economically interesting, e.g., using off-peak power or cogeneration to reduce or share costs. Small and medium power reactors based on high temperature gas reactors (HTGR) are also an attractive option. Future advanced nuclear power plants, such as the very high temperature reactors (VHTR) or the Supercritical Water Cooled Reactor (SCWR), could provide not only the electricity needed, but also deliver relatively high temperature process heat, providing high net power cycle efficiencies.
Steam reforming of natural gas1 is dominant in today’s refineries and the production of process heat by HTGR for the refining process could be a starting point towards a fossil-free (and nuclear generated) hydrogen production. In comparison to a conventional steam reformer, the employment of a nuclear steam reformer requires certain changes, since the operational conditions of a nuclear reactor are not as flexible as those of a fossil-fuelled furnace. Furthermore, safety requirements are much more stringent than for a fossil-fuelled system. Therefore, the highest effectiveness in the use of nuclear
process heat is required. A high hydrogen production rate is achieved if the process feedgas rate2 and the conversion rate3 are high.
Hydrogen Generation by Electrolysis
Electrolysis is the most straightforward process currently available to produce hydrogen directly from water (Fig. 1). Although conventional low temperature electrolysis can be coupled with all currently operating reactors, it will not be economically competitive. At higher temperatures, various potential processes for hydrogen production, such as high temperature steam electrolysis and other thermochemical processes have been identified. In this case, the use of high temperature reactors for hydrogen production presents a viable option since most of these processes have a higher efficiency than low temperature electrolysis. The types of electrolysis that are being considered for deployment on an industrial scale are, apart from the classical alkaline electrolysis, proton exchange membrane (PEM) electrolysis, and high temperature steam electrolysis (HTSE) using oxygen conducting ceramics.

FIG. 1. Standard low temperature alkaline electrolysis.
Electricity input required for electrolysis can be decreased by increasing the temperature range, as the total energy demand for electrolysis in the vapour phase is reduced by the heat of vaporization, which can be provided much more cheaply by thermal rather than electric energy. Indeed, in the high temperature range of 800–1000°C, electricity input could be about 35% lower than that of conventional electrolysis. In addition, the efficiency of electrical generation at these high temperatures is significantly better. The HTSE process is advantageous due to its high overall thermal-to-hydrogen efficiency when coupled with high efficiency power cycles.
As HTSE corresponds to the reverse process of a solid oxide fuel cell (SOFC)4 , respective devices could be operated in both modes. Therefore, HTSE development could benefit from ongoing R&D efforts in the SOFC area.
A high temperature electrolyzer must be coupled to a heat and power source. At high temperatures, all reactions proceed very rapidly. The steam–hydrogen mixture exits from the stack and then passes through a separator to separate hydrogen from the residual steam. The feedgas stream to the HTSE cell contains a 10% fraction of hydrogen in order to maintain reducing conditions and avoid the oxidation of the nickel in the hydrogen electrode (Fig. 2). HTSE cells can be operated at high current densities, which allow for large production capacities in comparatively small volumes. An electricity-tohydrogen efficiency of about 90% could be achievable. However, the lifetime of the hydrogen electrode, which is limited by degradation, requires further improvement.
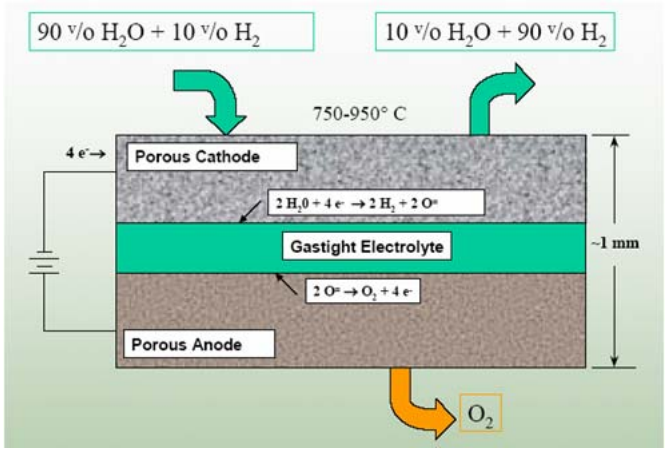
FIG. 2. Schematic of a planar steam electrolysis cell.
Hydrogen Generation by Thermochemical Cycles
Besides high temperature electrolysis, another promising candidate to produce large amounts of hydrogen by high temperature water-splitting is thermochemical process. The most straightforward method of water splitting would be a one-step direct thermal decomposition. However, this would require temperatures of > 2500°C for reasonable quantities, which is industrially not feasible. Therefore, multi-step processes are being considered.
A thermochemical cycle is a process consisting of a series of thermally driven chemical reactions where water is decomposed into hydrogen and oxygen at moderate temperatures. Supporting intermediate chemical compounds, which are regenerated and recycled internally and remain — ideally — completely in the system, are used in a sequence of chemical and physical processes. The only input to the cycle is water and high temperature heat. Therefore, these cycles are potentially more efficient than low temperature electrolysis and could significantly reduce production costs. Research on thermochemical cycles mainly focus on using solar or nuclear primary heat input.
Numerous thermochemical cycles have been proposed in the past and checked against factors such as: corrosion problems, cost analysis, heat transfer, material stability, maximum temperature, processing scheme, reaction kinetics, separation of substances, side reactions, thermodynamics, thermal efficiency and toxicity. Some have sufficiently progressed to be experimentally demonstrated and have already proven their scientific and practical feasibility. All cycles however, have design challenges and none has actually been implemented on a commercial scale.
A major challenge in thermochemical cycles is obtaining maximum yields while reducing the amount of excess reagents used to drive the reactions in the desired directions. Therefore, the optimization of heat flows is important for high energy conversion efficiency. One cycle under special consideration is the sulphur–iodine (S–I) process, also known as Ispra Mark 16 cycle, originally developed by the US company General Atomics and later taken up and modified by different institutions like the Japan Atomic Energy Agency (JAEA). This cycle basically consists of three chemical reactions (Fig. 3). Of all thermochemical cycles, the S–I cycle is the one with the highest efficiency quoted. The theoretical limit of efficiency for the total process is assessed to be 51%, assuming ideal reversible chemical reactions. Analytical studies anticipate efficiencies of 40–50%. As the result of a flow sheet analysis in 1982, General Atomics estimated the process thermal efficiency to be 47%. A schematic of the S–I cycle is shown in Fig. 3.
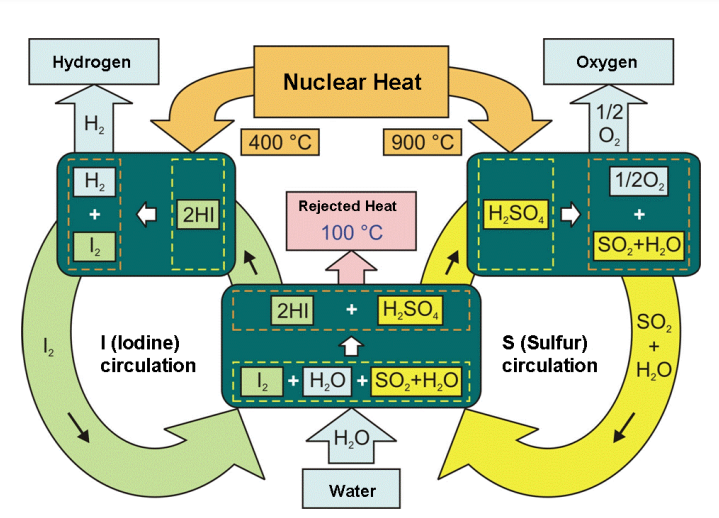
FIG. 3. Schematic of sulphur–iodine thermochemical water splitting cycle.
In all studies that systematically examined thermochemical cycles, those of the sulphur family — S-I, hybrid5 sulphur, sulphur–bromine hybrid — have been identified as the potentially most promising candidates, with higher efficiency rates and a lower degree of complexity (in terms of the number of reactions and separations). All three have in common the thermal decomposition of sulphuric acid at high temperatures (Fig. 4).
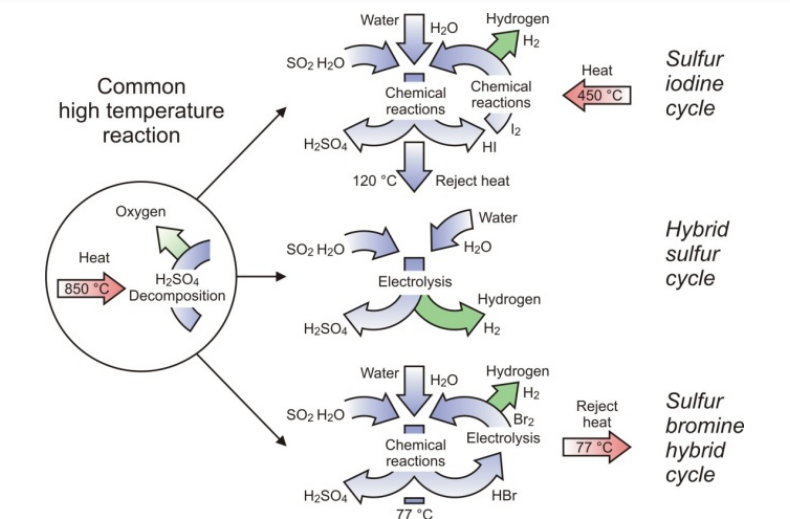 FIG. 4. Thermochemical cycles of the sulphur family.
FIG. 4. Thermochemical cycles of the sulphur family.
Hydrogen Generation by Hybrid Cycles
A hybrid cycle combines the benefits of thermochemical and electrolytic reactions. In this type of cycle, the low temperature reaction, which has a low thermodynamic efficiency and is therefore not favourable, is forced electrochemically. The hybrid-sulphur (HyS) process, originally studied at the Los Alamos Scientific Laboratory and further developed by Westinghouse in 1973–83, is also known as the Ispra Mark 11 cycle. It is a variation of the S–I process which consists of only two reaction steps and where sulphur, apart from hydrogen and oxygen, is the only other element involved. A series of flash evaporators are used to separate oxygen from the liquid mixture resulting in almost pure oxygen. The rest of the mixture, consisting of mainly SO2, sulphuric acid and water, is sent to the electro–chemical section. The mixture of SO2 and water is reacted in an electrolytic cell at lower temperatures to produce H2 and a sulphuric acid in an aqueous phase. Still, the net thermal energy requirement for the HyS process is significantly less than for conventional water electrolysis. SO2 electrolyzers require no more than 25% of the electricity that is needed in low temperature water electrolysis, i.e. ~0.29 V. Theoretically, this value could be decreased to 0.17V. Nevertheless, this would require that H2SO4 be decomposed at high temperatures in order to recycle the SO2 for the completion of the cycle. The Westinghouse process (Fig. 5) is simpler in design as the use of corrosive halides is not required. After the oxygen is removed from the system, the SO2 and H2O are combined with make-up H2O6 and routed to the electrolyzer cell. The SO2 is then electrochemically oxidized at the anode to form H2SO4, protons and electrons. The protons migrate through the electrolyte and produce H2 gas at the cathode.
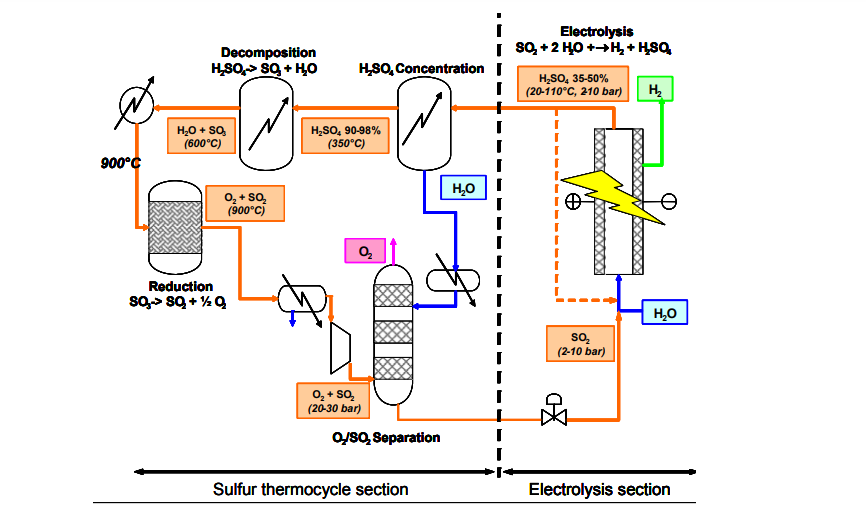
FIG. 5. Schematic of the Westinghouse hybrid-sulphur cycle.
Developed as the Mark 13 cycle at the Joint Research Centre of the European Commission in Ispra, Italy (JRC Ispra), the sulphuric acid–bromine (S–Br) hybrid cycle uses bromine instead of iodine. The S–Br hybrid cycle uses elements in only liquid or gaseous form. The voltage required in the electrochemical step (0.8 V) is slightly higher than in the HyS cycle (0.6 V) using this method. In 1978 in a lab-scale facility at the JRC Ispra, Italy, the cycle was successfully tested demonstrating a hydrogen production rate of 100 l/h over 150 h, with an efficiency of 37%. The system was also operated with a 1 kW solar heat source. However, reducing the energy requirement for the electrochemical step is currently the main area of focus.
Several alternative thermochemical cycles for hydrogen production, which operate at moderate temperatures in the range of 500–600ºC, have been investigated. Lower operating temperatures reduce the costs of materials and maintenance and can effectively use low grade waste heat, thereby improving cycle and power plant efficiencies. Additional advantages include ease of handling the chemical agents and reactions.
The US Nuclear Hydrogen Initiative (NHI) identified a number of chlorine-based thermochemical cycles. The copper–chlorine (Cu–Cl) cycle, shown in Fig. 6, can be operated at a maximum temperature of about 550°C. A hybrid cycle consisting of several thermal and one electrochemical reaction, the Cu–Cl cycle requires much lower operating temperatures than other cycles and can effectively use low-grade waste heat. The steps involved in the cycle are shown in the schematic below. Potential efficiency could reach as high as 41%. The electric energy demand was assessed at 39% of the total energy required. The viability of all reactions and, in particular, the H2 and O2 generation, was demonstrated at the Argonne National Lab on a bench-scale level. Nevertheless, side reactions and the completeness of the reactions are still being researched.
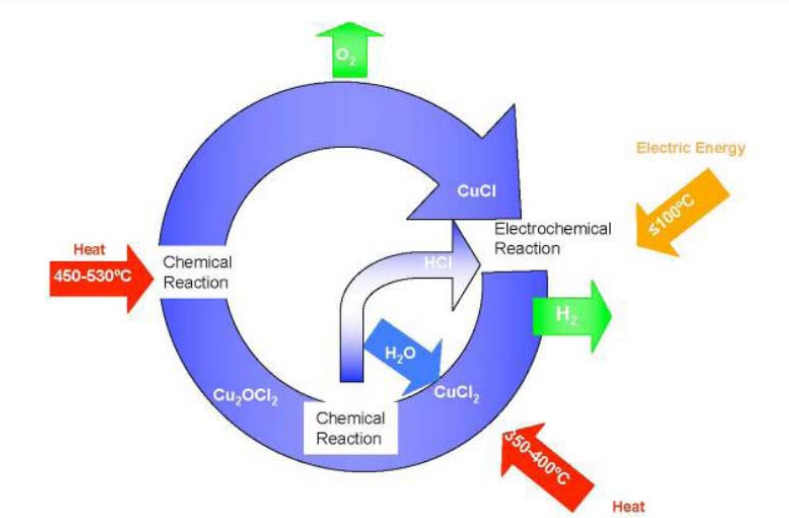
FIG. 6. Schematic of the copper–chlorine thermochemical water splitting cycle.
The iron–chlorine or “Mark 9” cycle is one of the thermochemical cycles that have been extensively studied in the past. Some partial reactions are well known and technically proven. The endothermic reaction is the hydrogen producing step by hydrolysis of FeCl2. The choice of proper material as well as the coupling to a process heat source still needs to be resolved. Also, separation of the solid and gaseous reaction products appears to be difficult, considering the melting point for FeCl2 (950°C) and its high vapour pressure at such high temperatures.
Other hybrid cycles also appear feasible. The hybrid copper oxide copper sulphate thermochemical cycle, originally derived from the Westinghouse HyS cycle, appears to be feasible with the technology currently available. Another promising thermochemical process is the calcium–iron–bromine or UT-3 cycle, developed at the University of Tokyo (UT) already some decades ago. The process consists of four gas–solid reactions which include hydrolysis and bromination of calcium and iron compounds.





