Nguyen Van Nhu , Truong Nhu Tung Institute of Energy and Climate, Juelich Center for Scientific and Technical Research, Germany Email: nguyen3vannhu@yahoo.com
Climate change and fossil fuel depletion are the main reasons for the emergence of new energy transition strategies - hydrogen strategies and hydrogen technology. As a very clean-burning fuel (producing only steam), hydrogen will play an important role in keeping the environment clean and ensuring energy security. Hydrogen is the main element that stores excess electricity. Hydrogen often exists in nature in compounds with other elements. Hydrogen technology is responsible for extracting hydrogen from other molecules in nature and using it as a carbon-neutral fuel as well as a very important raw material in industry and life. There are many processes for producing hydrogen from conventional and renewable energy sources. This article introduces the latest advances in clean hydrogen production technology using wind energy, solar power, biomass, biogas, etc. In which, the focus is on advances in electrolysis technology. water and seawater electrolysis, combined electrolysis and solar energy as well as synthetic fuel production.
Keywords: Hydrogen production, water electrolysis, seawater electrolysis, copper electrolysis, biomass, biogas, synthetic fuel.
Hydrogen is the lightest element on the periodic table and the most abundant chemical in the universe. On Earth, hydrogen is often found in complex molecules such as fossil fuels or water. As a very clean-burning fuel (producing only steam), hydrogen will play an important role in the transition to a zero-emission society c〇2 .
The current release of CO 2 into the atmosphere due to burning fossil fuels poses a serious threat to global climate change. On January 17, 2017, 13 leading transportation and energy industry companies at the World Economic Forum in Davos (Switzerland) launched a global initiative called the “Hydrogen Council” to talk about to a unified vision and long-term ambition for hydrogen to drive the global energy transition.
Hydrogen is a raw material/fuel for industry and energy conversion. Hydrogen and hydrogen-rich fuels such as natural gas and biogas can be used in fuel cells to cleanly and efficiently provide electrical and thermal energy in a variety of mobile power and energy applications. permanent. Hydrogen is a smart solution for the sustainability of future energy systems because hydrogen can be used as an energy carrier and storage medium in smart grids and other new applications. The widespread deployment of hydrogen and fuel cell technologies brings many benefits to the environment, ensuring energy security for the domestic economy and for users.
Global hydrogen demand is about 90 million tons of H2 in 2020, up 50% since the turn of the millennium. Most of this demand comes from oil refining and industrial use in chemical production such as ammonia, methanol, and reducing agents in steel production [1]. There are many processes to produce H 2 according to the raw material. used. Can be divided into two main categories: conventional technology and renewable technology.
The first type of fossil fuel processing is comprised of hydrocarbon reforming and pyrolysis methods. These methods are the most developed and most commonly used to cover almost all hydrogen demand. Specifically, to date hydrogen is produced 48% from natural gas, 30% from heavy oil and naphtha and 18% from coal [2] [3]. In the hydrocarbon reforming process, there are mainly steam reforming (SR), partial oxidation (POX) and automatic thermal steam reforming (ATR) processes.
The second category includes methods for producing hydrogen from renewable resources or from biomass or water. Using biomass as feedstock, these methods can be divided into two general subcategories, thermochemical and biological processes. Thermochemical technology mainly includes combustion gasification pyrolysis and liquefaction while the main biological processes are direct and indirect biophotolysis dark photofermentation and dark fermentation and fermentation sequential optical enamel. The second type of renewable technology involves methods that can produce H 2 through water splitting processes such as pyrolysis, electrolysis, and photoelectrolysis that use water as the sole input material.
Potential pathways for the production of hydrogen and hydrogen-based products are presented in Figure 1.
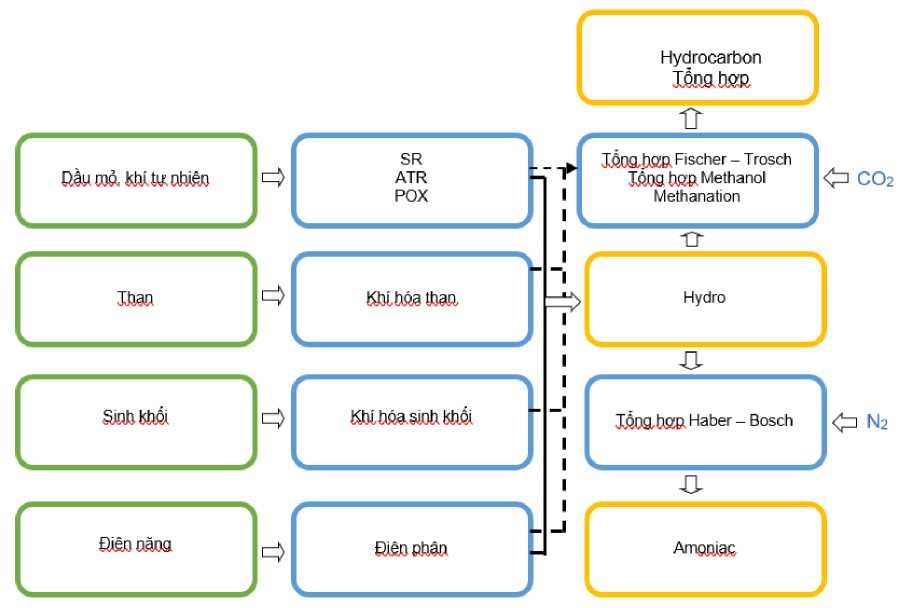
Figure 1. Potential pathways for the production of hydrogen and hydrogen-based products [4]
To extract hydrogen from other molecules in nature and use it as a carbon-neutral fuel, additional energy is required, and the energy used in this process needs to be renewable to impact the overall climate. can become neutral.
In recent years, colors have been used to indicate different sources of hydrogen production. Gray refers to hydrogen production from natural gas, black or brown are from coal and brown coal respectively. Blue is commonly used to produce hydrogen from fossil fuels with reduced c〇2 emissions due to the use of c〇2 burial (CCS) techniques or the application of c〇2 conversion for fuel production other material. Green is a term applied to hydrogen production from renewable electricity. In general, there are no established colors for hydrogen from nuclear power, biomass or different types of grid power.
The main hydrogen production processes (both technical and economic aspects) are summarized in table 1.
| Method | Efficiency (%) | Main advantages | Main disadvantage |
| SR | 74-85 | The infrastructure is there and the technology is most developed | CO2 byproduct depends on fossil fuels |
| POX | 60-75 | The infrastructure is there, the technology is proven | CO2 byproduct depends on fossil fuels |
| ATR | 60-75 | The infrastructure is there, the technology is proven | CO2 byproduct depends on fossil fuels |
| Methane pyrolysis | - | No CO2 emissions, reduced process steps. | Black coal is a byproduct, dependent on fossil fuels. |
| Biomass pyrolysis | 35-50 | Abundant and cheap raw material supplies are CO2 neutral. | Formation of tar, H2 yield varies, capacity varies with season and feedstock impurities |
| Gasification of biomass | - | Abundant and cheap raw material supplies are CO2 neutral. | Formation of tar, H2 yield varies, capacity varies with season and feedstock impurities |
| Biophotolysis | ten | Consuming CO2, O2 is the only by-product that operates under mild temperate conditions | Requires sunlight, low H2 rate and output, requires large volume reactor, Sensitivity to O2, high raw material cost. |
| Ferment in the dark | 60-80 | CO2-neutral, simple, can produce H2 without light, contributing to waste recycling without limiting O2. | Fatty acids must be removed, H2 rate and yield are low, requiring large reactor volumes. |
| Fermentation under light conditions (photofermentation) | 0.1 | CO2-neutral, contributes to waste recycling, can use various organic wastes and wastewater | Requires sunlight, low H2 rate and output, low conversion efficiency, requires large reactor volume, sensitive to O2 |
| Electrolysis of water | 40-60 | Non-polluting with renewable sources, proven technology, established infrastructure, abundant feedstock, O2 as the only by-product, contributing to the integration of RES as an electricity storage option . | Overall efficiency is low, capital costs are high |
| Thermolysis | 20-45 | Abundant, clean and sustainable raw materials, O2 is the only by-product | Elemental toxicity, corrosion problems, high capital costs |
| Photo-electrolysis by light (photo-electrolysis) | 0.06 | Abundant raw materials, no emissions, O2 is the only by-product. | Requires sunlight, low conversion efficiency, ineffective photocatalytic materials |
In terms of climate impact, the most promising hydrogen production is water electrolysis. During this production process, water is split into hydrogen and oxygen. However, to produce large quantities of hydrogen, industrial-scale installations are required that use large amounts of electricity and operate at varying efficiencies.
Directly connecting renewable energy (wind power, solar power, hydropower) with hydrogen production is the best way to ensure green hydrogen production with almost zero c〇2 emissions. If we use electricity generated from fossil fuels, it will increase CO2 emissions instead of reducing them.
Electrolysis is the core technology of Power to X solutions where X can be hydrogen, syngas or synthetic fuel [5] [6] [7]. When electrolysis is combined with electricity from renewable sources, the production of fuels and chemicals can be decoupled from fossil resources, paving the way for an energy system based on 100% renewable energy. .
With advanced technologies developed and artificial intelligence applications, we need to change the course of action in solving future energy problems intelligently.
Figure 2 shows how renewable energy sources can participate in a large national system to produce renewable energy and renewable electricity for industrial, transport and residential needs.
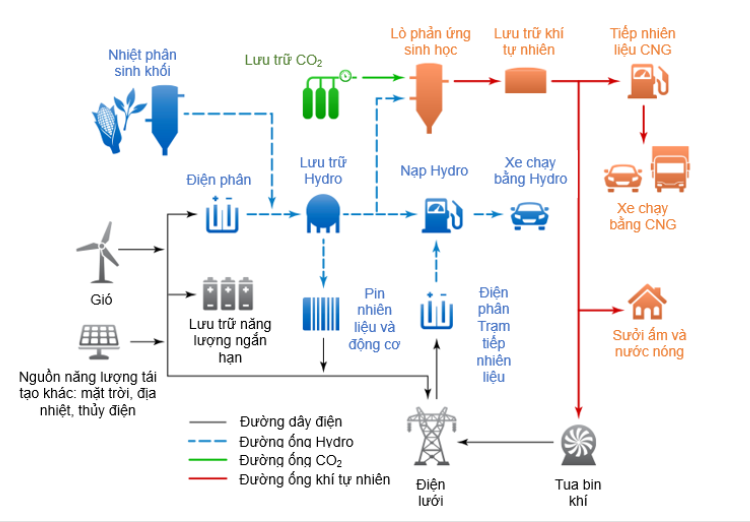
While hydrogen has the highest gravitational energy density (kWhkg) of all chemicals, the volumetric energy density (kWhlitre) of hydrogen at ambient temperature and pressure (15°C and 0.1 MPa ) is very low. Therefore, to facilitate long-range storage and transportation of larger volumes, hydrogen needs to be modified or converted into a higher volume density form. The most suitable options are high pressure regulation around 1000 bar (compressed gaseous hydrogen CGH2), low temperature liquefaction (liquefied hydrogen LH2) at -253 °C, using a liquid organic hydrogen carrier (Liquid Organic Hydrogen Carriers: LOHC) and converted into a chemical with higher density such as NH3.
The role of hydrogenation in future smart and sustainable energy systems can be summarized as follows [9]:
a) Enable efficient integration of renewable energy on a large scale: Due to the unstable nature of renewable energy sources, the supply and demand sides need to be balanced. Hydrogen can be used to store renewable energy when it is in surplus in the form of chemicals and can then be used to meet demand during shortages;
b) Transportation and distribution of renewable energy between sectors and regions around the world: Hydrogen can be used to convert renewable energy sources into chemical energy and transport that energy to regions. Sectors and sectors effectively differ according to the supply and demand sides;
c) Hydrogen storage to increase strategic energy storage capacity: Hydrogen has a high energy density that can be stored for long periods of time and is easy to transport. It is therefore well suited to serve as an energy buffer and strategic energy reserve;
d) Reducing CO2 emissions in transportation: Hydrogen-powered vehicle technologies are expected to make CO2-free transportation possible even for heavy trucks and vehicles. fire and ships;
e) Reducing CO2 emissions in industrial energy use: Industries can use a mixture of electricity and green hydrogen to produce high-temperature steam needed for many processes.
industrial process. Applying Hydrogen to directly reduce iron ore to replace coal in steel production;
f) Acting as a raw material for industry: Carbon from biomass and green hydrogen are the main raw materials for the production of many bulk chemical products;
g) Helps reduce emissions in home heating or cooling systems: Heating systems need to integrate energy sources with new technologies such as heat pumps. Natural gas can be partially replaced by sustainable hydrogen transported through existing gas pipelines.
This article introduces the latest advances in clean hydrogen production technology and hydrogen-based synthetic fuels in the world in the trend of developing smart energy systems.
2 Electrolysis technology
The continuous increase in the share of renewable but unstable energy sources (such as wind power, solar power) will greatly change the energy grid where water electrolysis begins to play an important role in the storage of gigawatts of energy in the form of hydrogen (power to H2). This is already a reality that needs to be addressed in countries such as Germany and Denmark, where there have been several large-scale demonstration projects on water electrolysis to store excess wind energy (especially at night). Excess solar electricity on hot days is in the form of Hydrogen chemicals.
Figure 3 presents the three most popular electrolysis technologies with their respective partial reactions for the hydrogen evolution reaction (HER) and the oxygen evolution reaction. Where both alkaline and acid electrolysis equipment (PEM) require liquid water to solvate the ions to pass through the diaphragm (alkaline) or membrane (PEM). For solid oxide battery cells, O-2 transport through a dense ion conductor consisting of Zr02 doped with Y2O3 occurs only between 650 and 1000°C.
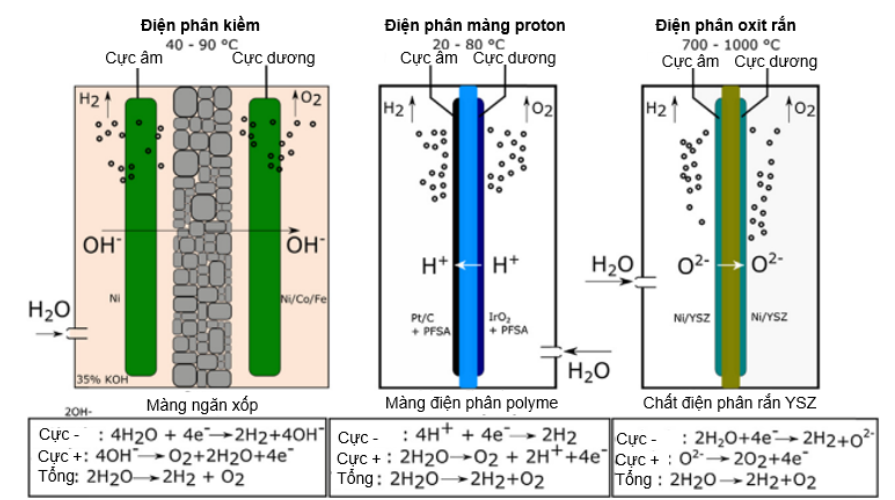
Figure 3. Operating principles of different types of water electrolysis [10]
As equipment, alkaline electrolysis is the most mature electrolysis technology, so they dominate the market especially for large-scale projects (both operational and announced). Alkaline electrolysis uses an electrolyte that is an aqueous solution containing about 25-35% by weight of KOH usually running at 80-90°C from normal pressure to high pressure up to 200 bar. Only materials that can withstand harsh conditions are selected which are usually steel or asbestos- Ni0- or Zr02 electrode shields and potassium hydroxide waterproofing polymer materials for the frame and or gasket.
However, many new projects are now choosing polymer electrolyte membrane (PEM) designs. PEM electrolyzers can operate more flexibly and are therefore more compatible with applications using unstable renewable electricity. For the PEM type, the acidic conditions provided by the perfluorosulfonic acid membrane and ionomer and the high potential difference on the anode side (oxygen evolution) will require the use of iridium and platinum-based noble metals. Pt needles and use of titanium-based components. Catalytic requirements are a major barrier for this technology. In the PEM electrolyzer, 300 kg of platinum and 700 kg of iridium are required.
per GW. Therefore, if PEM supplies all electricity generation by 2030 in a zero CO2 emission scenario, demand for iridium will skyrocket to 63 kt, nine times current global production [1].
Solid oxide cell electrolysis (SOEC) technology is attractive because of its very large conversion efficiency of 79% - 84% (LHV) which results from favorable thermodynamics and kinetics at higher operating temperatures. The SOEC solid oxide electrolyzer requires nikgn (150-200 t/GW), zirconium (40 t/GW), lanthanum (20 t/GW) and yttrium (5 t/GW). Over the next decade it is expected that new advances in design will halve each of these amounts, with the potential for new techniques to reduce nickel content to less than 10 t/GW [1].
Electrolyzer users are left wondering whether the operational benefits of PEM (flexibility) and SOEC (efficiency) are worth the additional cost compared to alkaline electrolyzers.
Hydrogen production technology trends using water electrolysis aim at the strategy of increasing performance, durability and reducing costs. An overview of research and implementation of water electrolysis technology can be found in documents [1][10][11] [12] [13] [14][15].
Electrolyzers can also be operated in reverse mode as fuel cells to convert hydrogen back into electricity [16]. Combined with hydrogen storage facilities, they can provide balancing services to the grid to increase overall equipment utilization rates. The latest example of a solid oxide reversible fuel cell system with a capacity of 5 kW has been successfully tested [17].
Co-electrolysis of H2O and CO2
SOEC can be used to directly electrochemically convert water vapor (H2O), carbon dioxide (CO2) or both by a “co-electrolysis” process to hydrogen (H2), carbon monoxide ( CO) or syngas (H2 + CO) respectively) to then convert into synthetic fuel [5] [6].
For co-electrolysis of H2O and CO2, in addition to the reactions occurring on the electrode, there are also water-gas shift reactions (WGS) and methanation reactions or direct steam reforming reactions. can occur in the porous fuel electrode (cathode) [6]. The two overall equations of the co-electrolysis of H2O and CO2 are represented in equations (1) and (2), the water-gas displacement reaction is a reversible reaction represented in equation (3).
|
H2O (g/l) + electrical energy + heat → H2 + ½ O2 CO2 + electrical energy + heat → CO + ½ O2 CO + H2O ⇌ CO2 + H2 |
(first) (2) (3) |
In the latest research [18] [19] it has been shown that co-electrolysis of a mixture of H2O and CO2 (Coelectrolysis at high temperature (700 - 800 °C) can create syngas products (H2 + CO ) is specifically designed in the area relevant to downstream catalytic processes.
Syngas composition is determined mainly by the H2O and CO2 input ratio. During co-electrolysis, mainly water vapor is electrochemically reduced to hydrogen with a Faraday efficiency close to 1. CO2 gas is reduced by reaction. reverse water-gas reaction from hydrogen to CO. The product gas composition is consistent with the thermodynamic equilibrium of the reaction (3). The CO:H2 ratio can be increased by adding CO2 or decreased by adding steam to the co-electrolyzer.
Currently, many medium-sized and quite large businesses are emerging in the field of water electrolysis equipment to prepare for the emergence of the hydrogen market. German company Sunfire has offered products on Alkali electrolysis equipment (Sunfire-HyLink Alkaline) and SOEC (Sunfire-HyLink SOEC) for testing and research and development [20]. Siemens, Thyssenkrupp, Nel Hydrogen ITM McPhy Cummins and John Cockerill develop PEM technology on a large scale and have announced plans to expand their production capacity. Haldor Topsoe builds a large-scale SOEC electrolyzer manufacturing facility to meet customer demand for green hydrogen production [21]. The plant will have an electrolyzer production capacity of 500 MW per year and can expand to 5 GW. The product will be an industrial-scale electrolyzer based on Topsoes' proprietary SOEC high-temperature electrolysis technology that provides 30% greater hydrogen yield than standard technology such as PEM and alkaline electrolysis. This production facility will go into operation in 2023.
Global capacity of electrolysers to produce hydrogen from electricity has doubled over the past five years and was more than 300 MW by mid-2021. About 350 projects are currently under development that could deliver global capacity up to 54 GW by 2030. Another 40 projects accounting for more than 35 GW of capacity are in the early stages of development [1].
Water electrolysis technology advances are gradually improving their key performance parameters such as energy efficiency and flexibility to respond to varying electrical loads. PEM electrolysis requires rare and expensive metal catalysts. To reduce costs, Carmo and his colleagues have successfully researched a recycling solution to recover rare metals such as Ir and Pt from used ion exchange membranes [22]. Hegge and colleagues applied nanofibers to create an interlayer in the anode to reduce the amount of Iridium while still achieving efficiency for PEM water electrolysis [23].
A large research project bringing together many scientists and technologists from universities and industrial firms of the Federal Republic of Germany called Kopernikus P2X (Power to X) is working very actively. The project's objective is to develop the technological base for renewable energy exploitation solutions to produce chemical energy storage systems such as fuels (from electrolysis: electrofuels) and chemical products. quality in accordance with economic and social requirements.
The overarching structure of the Kopernikus P2X project is divided into three phases, ranging from basic research to applied research and finally implementation research leading to technical demonstration.
In the second phase of the Kopernikus P2X project (P2X II), 42 partners are continuing to develop technologies in different value chains with the aim of bringing them to market. The two energy vectors that have been identified, hydrogen and syngas, can be used as a basis to create dedicated value chains for three application areas of the transport and basic chemicals industries from electrolysis to manufacturing. final product and or final application. An overview of early research results is presented in the document [24]. Five main results have been obtained including:
- Minimizes the amount of rare metal Iridium catalyst needed for water electrolysis without affecting performance;
- Master the process of electrolysis of mixtures of c〇2 and water (Co-electrolysis) with different CO2/H2O ratios;
- Research, develop and put into operation a 4-step continuous equipment system to produce 10 liters/day of liquid fuel from renewable electricity;
- A cheap and effective catalyst for the dehydrogenation of LOHC (liquid hydrogen storage) has been found;
- Successfully produced low CO2 emission fuel Oxymethylenether (〇ME) from H2, CO and Methanol with a cheap catalyst that does not contain rare metals.
The use of high-cost electrocatalysts composed of rare earth metals including iridium ruthenium and platinum makes PEM water electrolysis a less attractive option. Project RENEW under the EU-funded HORIZON program aims to change this by conducting research and documenting water oxidation catalysts to develop a cheaper catalyst that uses many abundant metals like iron, cobalt and nickel. The findings of this project could have the potential to transform energy sectors worldwide [25]. Hopefully in the coming years the project will produce the desired results.
Electrolysis of sea water
Electrolysis of water to create hydrogen fuel is an attractive renewable energy storage technology. However, large-scale freshwater electrolysis would severely strain critical water sources.
The most serious challenges in seawater separation are posed by chloride anions (0.5 M in seawater). Under acidic conditions, the equilibrium potential of the OER relative to the normal hydrogen electrode (NHE) is only 130 mV higher than the chlorine evolution. Even with highly active OER catalysts in alkaline electrolytes, chloride anions in seawater can aggressively corrode many catalysts and substrates through the mechanism of metal chloride-hydroxide formation in the following ways: Procedures (4), (5) and (6) [26]:
Cl- adsorption by surface polarity
M +Cl- → MCL ads + e-
Combine further with Cl -
MCl ads +Cl- → MCl-
Conversion from chloride to hydroxide
MClx- +OH- → M(OH)x +Cl-
To avoid reliance on costly desalination processes, the development of corrosion-resistant electrodes to split seawater into H2 and O2 is crucial to the advancement of seawater electrolysis, a work that has yet to be completed. great success until recently [12].
Kuang et al [26] Stanford University developed a multilayer anode consisting of a nickel hydroxide (NiFe) electron catalyst layer uniformly coated on a nickel sulfide (NiSx) layer formed on porous Ni foam (NiFe /NiSx-Ni) for stable and active seawater electrolysis. Homogeneously electrodeposited NiFe、is a highly selective OER catalyst for splitting alkaline seawater while the underlying NiSx layer creates a conductive interlayer and a sulfur source to create a rich anode Stable cation-selective polyatomic anion against chloride corrosion. The seawater electrolyzer can achieve a current density of 400 mA/cm2 under 2.1 V in real seawater or salt-accumulated seawater at room temperature while requiring only 1.72 V under industrial electrolysis conditions. industrial at 80 °C.
The authors coupled an activated NiFe/NiSx-Ni anode with a highly reactive Ni-NiO-Cr2O3 cathode (HER) for two-electrode alkaline seawater electrolysis. Three-electrode linear sweep voltammetry of Ni-NiOCr2O3 showed that an overpotential of 0.37 V was necessary to generate a HER current density of 500 mA/cm2. The results of running at 400, 800 and 1000 mA/cm2 all show that the oxygen generation efficiency is nearly 100%.
In real seawater electrolysis applications, salt can accumulate in the electrolyte if seawater is continuously fed into the system and the water is converted to H2 and O2. To achieve this goal, the authors studied electrolytes with higher NaCl concentrations in seawater using deionized water with 1 M KOH +1 M NaCl or even +1.5 M NaCl . The electrolysis remained stable for over 1000 hours with no obvious corrosion or voltage rise showing that the anode was functional and impressively stable for electrolysis in high salinity water. A device with such material offers the opportunity to use Earth's vast seawater as an energy carrier.
3 Hydrogen-based synthetic raw material/fuel production technology (Power-to-X=fuel)
The conversion of H2 and CO2 to liquid feedstock/fuel (X=Liquid) through Power-to-Liquid (PtL; X=Liquid) processes is gaining attention because of the higher energy density of liquid fuel feedstocks. Compared to gas and easier to store and transport. PtL enables field connectivity by capturing and utilizing CO2 and can be used to produce valuable products for the chemical industry. For the synthesis step, the major challenges are the direct use of CO2 and flexible and dynamic operations to improve design choices.
Electrolysis is the core technology of Power to X solutions. Figure 4 illustrates the integration of SOEC with methane, methanol, and ammonia synthesis and the beneficial use of heat from exothermic synthesis processes. The released heat can be used to generate the steam needed as feedstock for SOEC.
SOEC can be thermally integrated with a variety of chemical syntheses allowing the recycling of captured CO2 and H2O into synthetic natural gas or methanol gasoline or ammonia, leading to further efficiency improvements compared to other technologies. electrolysis at low temperature [11].
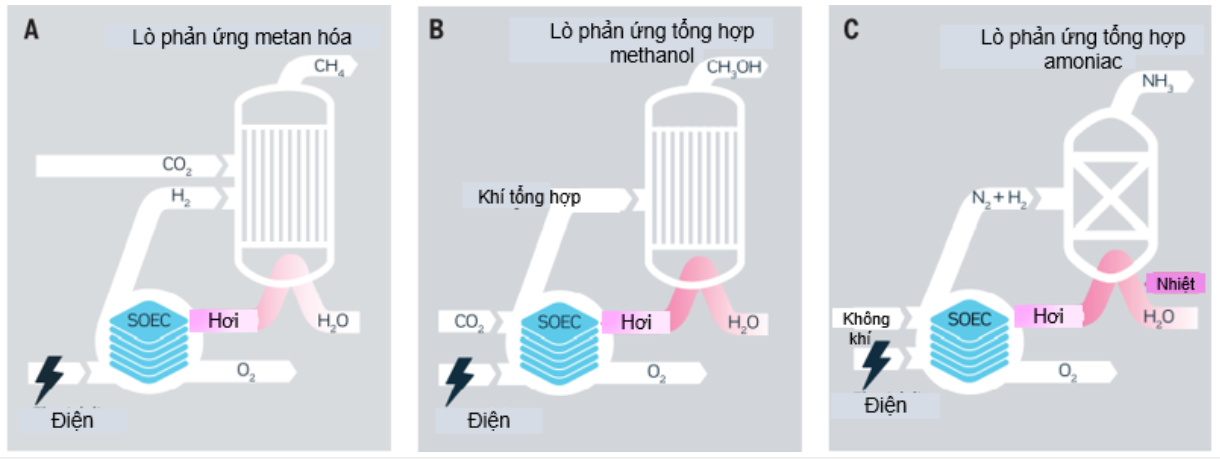
Figure 4. Integration of solid oxide electrolysis system with chemical synthesis [11]
Note in Figure 4C that in conjunction with ammonia synthesis, the unique ability of SOEC to act as an oxygen barrier that utilizes heat instead of power can be exploited to eliminate the the need for expensive air separators to supply nitrogen.
Schemme et al. [27] technically compared a number of different Power-to-Fuel production options with respect to technological advancement and efficiency as well as cost. fee. Options examined included methanol, ethanol, butanol, octanol, DME,〇ME3-5 and hydrocarbons (figure 5). The results show that H2 production costs account for 58-83% of the total synthetic fuel production costs. Therefore, the ability to reduce hydrogen production costs in the near future will have a decisive influence on reducing product costs.
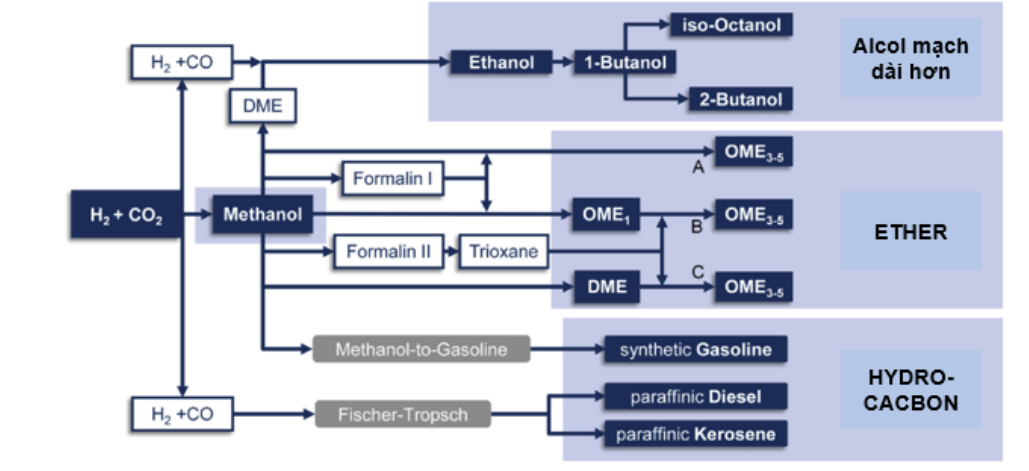
Figure 5. Some Power-to-fuel synthetic fuel production options [26]
Co-electrolysis is particularly interesting for PtL processes using syngas and the application of co-electrolysis has been discussed for methane, methanol, DME and Fischer-Tropsch-hydrogen carb/n fuels [27] [28] [29] and [30].
Direct synthesis of methanol from CO2-based gas has been tested in PtMeOH (Power to MeOH) pilot plants and even on a commercial scale. For direct and two-stage DME synthesis from C〇2 has been investigated, however pilot plants and optimal process routes are still lacking. Finally, several PtFT (Power to Fischer-Tropsch) fuel pilot plants based on FT synthesis were carried out but all included conversion from CO2 to CO. The direct use of CO2 in FT synthesis is in its early stages and further catalyst development and laboratory-scale testing are needed [31].
However, PtL poses new challenges for synthesis from large-scale continuously running plants based on syngas towards more flexible small-scale concepts with direct CO2 utilization. Current pilot plants and future concepts indicate that the development of integrated concepts for modular small-scale PtL prevails allowing decentralized operations close to sources renewable energy and CO2 sources. This trend is especially evident for FT-based PtL concepts [31].
Overall, commercialization of PtL will require further advances in product synthesis along with improvements in electrolysis and carbon capture.
The world's largest Power-to-X plant for the production and liquefaction of green hydrogen with a capacity of 24 megawatts when operational is now taking shape at the Leuna refinery and chemical plant site in Saxony-Anhalt, Germany. The electrolysis plant is initially planned to produce up to 3,200 tons of green hydrogen per year with the help of green electricity (renewable energy) generated nearby from mid-2022 [32].
A model of the first green hydrogen production system using wind power called Energiepark Mainz (Mainz Energy Park) has been built and operated continuously for many years in Mainz, the capital of the German state of Rheinland-Palatinate. A very impressive example of progress in research and deployment of smart energy networks. For the past 2 years, this system has completely connected the stages of green hydrogen production using electricity from 4 wind turbines using water electrolysis method, then the hydrogen product is used or used as additional fuel in the gas system. readily available or used as a fuel for storage and transportation to the point of consumption. The PEM electrolysis system can start up for continuous operation of 4 MW in just 15 seconds, which is very dynamic. The total plant efficiency is 70.4 % [33].
4 Hydrogen production using sunlight (Solar hydrogen production)
Promising technologies for producing hydrogen from water and sunlight energy include photovoltaic production (PEC) and photovoltaic-electrolysis (PV-EL) pathways. The difference between the two approaches is in the level of integration of the subsystems.
PEC combines photovoltaic and electrolytic effects in a single device. Light absorbed by a photoelectrode causes charge carriers to form an electric potential with an electrolyte junction, where water splitting occurs. The single-junction approach to generating hydrogen without an external power source, using titanium dioxide (Ti〇2) and platinum (Pt), was first demonstrated in 1972 by Fujishima and Honda.
Recently Cheng and colleagues [34] presented a monolithic photovoltaic device using Rh and Ti〇2 to split water directly with an efficiency of 19%.
In contrast to a PV-EL connector, the photovoltaic carriers are separated by a semiconductor-semiconductor junction (both are solids) and are typically transported via wires to a point. separate solid-liquid interface, where water separation by electrolysis takes place. A typical PV-EL combination is a combination of commercial PV modules and electrolyzers connected directly or connected via converters. Lab-scale PV-EL has achieved efficiencies greater than 18%. Theoretical calculation can be up to 41% [35].
Jia and co-workers presented a system consisting of two PEM polymer membrane electrolysers connected in series with a solar cell at an InGaP/GaAs/GaInNAsSb triple junction that generates a voltage large enough to drive both electrolytic cells. manure without additional energy input (Figure 6). The solar concentration is adjusted so that the peak power point of the photovoltaic matches the operating power of the electrolyzer to optimize system efficiency. The system achieved an average 48-hour STH efficiency of 30%. These results demonstrate the potential of photovoltaic-electrolysis systems for cost-effective solar energy storage [36].
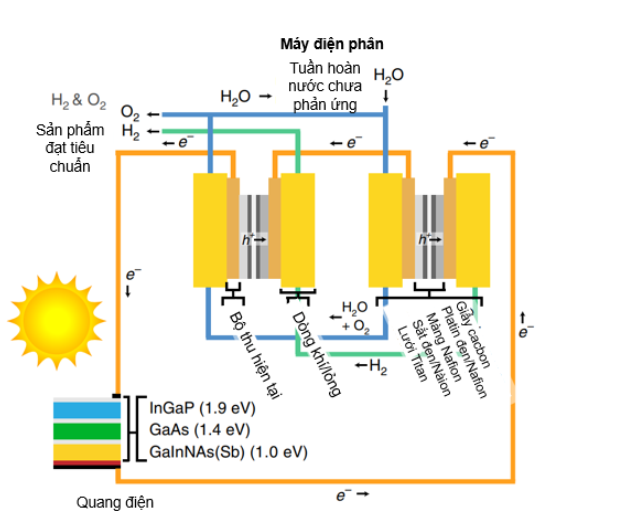
Figure 6. Diagram of PV electrolysis device. The PV electrolysis system consists of a three-junction solar cell and two PEM electrolyzers connected in series [36]
Recently, a scientific team from Utrecht University, the Netherlands, analyzed the technical economics of two solar-powered hydrogen production technologies, the photovoltaic system (PEC) and its main competitor, the solar energy system. The photovoltaic system is connected to a conventional water electrolyser (PV-EL system). A comparison between the two was made to determine the more promising technology based on equal cost of hydrogen (LCOH). The technical assessment was performed by reviewing proven designs and materials for the PV-EL system and for the PEC system extrapolated to future commercial scale. The LCOH for the off-grid PV-EL system was found to be 6.22$/kgH2 with a solar-to-hydrogen efficiency of 10.9%. For a PEC system with a similar efficiency of 10%, the calculated LCOH is much higher, specifically 8.43$/kgH2. The work has demonstrated the versatility of use, the PV-El system can be connected into future energy systems more flexibly and more efficiently than the PEC device because PV and EL can be optimized be specifically sized to suit the needs of future energy systems and grids [37].
The SUN-to-LIQUID project under the EU-funded HORIZON program aims to demonstrate a pre-commercial scale solar liquid fuel production facility in Mosteles Spain. The development of solar reactor and power supply technology is critical to the success of the project. The super-modular heliostats are densely arranged in front of the 15 m high solar tower and can emit concentrated solar radiation in excess of 3000 kW/m2. The reactor consisting mainly of a ceria insulated porous ceramic (RPC) structure was successfully scaled up from 4 kW to 50 kW. In the solar tower, a flux measurement system combined with a water calorimeter is used to calibrate the method of determining the solar energy entering the solar reactor and quantifying the power supply [38 ].
At the Sun-to-LIQUID solar plant in Mósteles near Madrid, scientists have for the first time succeeded in producing kerosene from the raw materials of water, carbon dioxide and concentrated sunlight in actual conditions. The plant consists of two steps, first syngas - a mixture of hydrogen and carbon monoxide - is produced using a mirror field and a solar tower with a solar thermochemical reactor based on the zero ceria redox reaction polarization. In the second step, a connected Fischer-Tropsch plant converts syngas into liquid kerosene [39].
5 Hydrogen production from biomass
Biomass is one of the main renewable sources for energy production. It can range from several sources such as agricultural waste, forest waste, waste from various industries and household and municipal waste. Converting biomass into energy can be done in many different ways, for example producing biogas, hydrogen, ethanol and biodiesel. Biomass acts as a natural battery that stores the sun's light energy in the form of chemical bonds unless it is not harnessed. The two main ways to produce hydrogen through biomass are thermal, chemical and biological methods [40]. Thermochemical processes include pyrolysis, gasification and liquefaction. Hydrogen production through biological pathways is renewable and environmentally friendly in nature. Many types of biomass can be used to generate hydrogen, for example agricultural residues, organic wastewater, municipal solid waste. Dark fermentation, photolysis, direct photolysis, indirect photolysis and microbial electrogenerating cells (MECs) are different methods to convert biomass into hydrogen.
5.1 Hydrogen production from carbohydrate foods
Biomass carbohydrates can be converted into hydrogen (equation 7) using biocatalysis, chemical catalysis and their combination (Figure 7). Chemical catalytic reactions are carried out under harsh reaction conditions, while biocatalysis is carried out under mild reaction conditions.
C6H10O5 +7H2O 一 12 H2 + 6 CO2 (7)
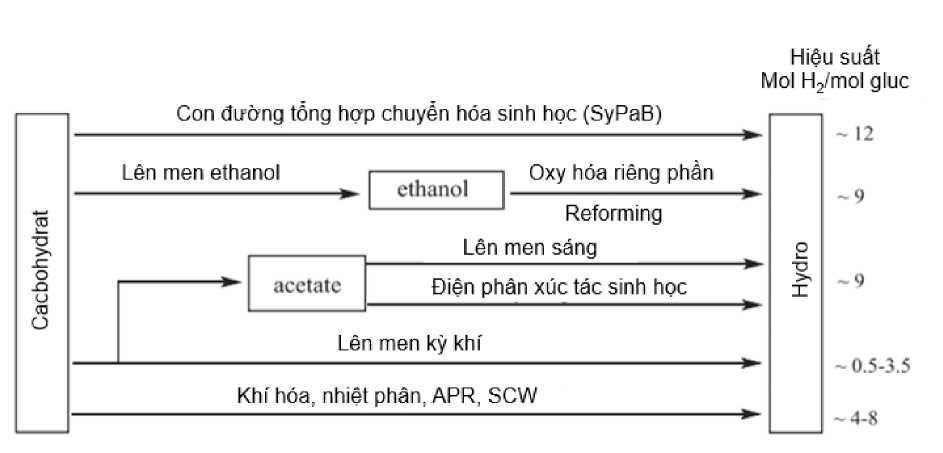
Figure 7. Comparison of hydrogen production yields from biomass carbohydrates using different approaches [41]
Different technologies have been used to obtain hydrogen from carbohydrates using biocatalysis such as anaerobic fermentation, ethanol fermentation, partial oxidation and cell-free SyPaB.
Biocatalysis is catalysis mediated by living systems such as microorganisms or protein enzymes at atmospheric pressure and ambient temperature. Biocatalysis has the advantage of cost-effective bioreactors, low energy input and high selectivity. However, the reaction rate of biological catalysts is lower. Biodialysis is a cost-effective way to produce H2 from organic sources with high water content, such as sewage sludge...
The cost of producing hydrogen from carbohydrates depends on its yield. Among them, the highest hydrogen yield at low cost was obtained from cell-free SyPaB. Additionally, the pathway design of cell-free SyPaB has several advantages such as the use of less expensive bioreactors or bioreactors, mild reaction conditions and acceptable reaction rates. . To achieve optimal performance, reactors can be arranged in parallel or in series sequence. can perform coupled or uncoupled reactions and can be of any shape and size.
5.2 Hydrogen production from non-food biomass
Zhang and colleagues recently described a dehydrogenation pathway that can be applied to non-food biomass and everyday waste such as straw, wheat, corn, rice straw, sugarcane bagasse, and cardboard mulch. Bamboo saw and newspaper. H2 yields of up to 95% were achieved by a two-step one-pot reaction with a 69 ppm molecularly defined iridium catalyst bearing the imidazoline moiety from formic acid, which in turn was obtained via a 1% mass promoted hydrolysis reaction. dimethyl sulfoxide accumulation of biomass [42]. The H2 production process diagram is presented in Figure 8.
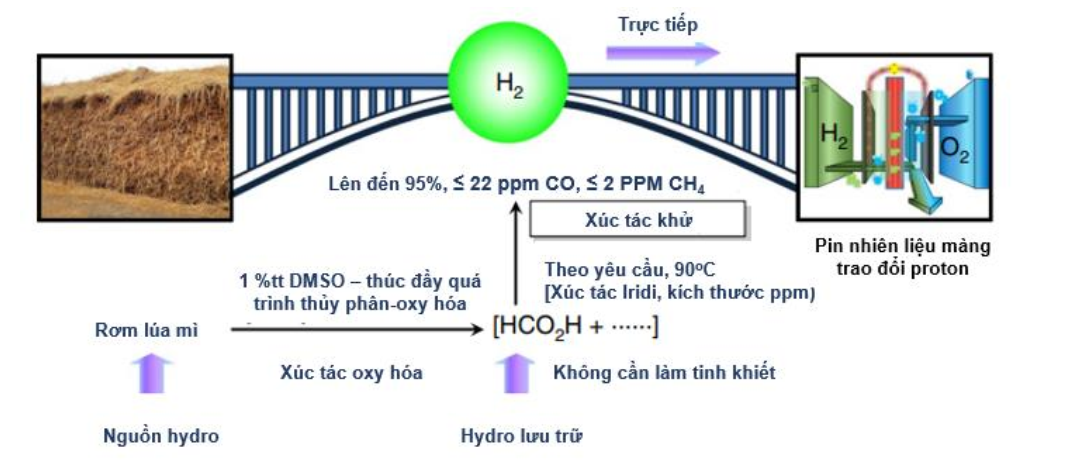
Figure 8. Streamlining the conversion of non-food biomass to electricity via H2 [40]
The first step of oxidative hydrolysis of various biomasses. Biomass was introduced into a solution containing HOAc, MeOH, DMSO, DMS〇2,1,4-dioxane, NU2SO4 and NaV03 HC02H (pH =2.25).
The second step of the H2 production process. The resulting hydrolytic oxidation mixture is transferred to the reaction vessel. Aqueous NaOH solution (10.0 ml 1) was added to the reaction mixture to the specified pH value. The reactor is then connected to the H2 producer.
The H2 produced is fed directly into the fuel cell. The fuel cell was run for more than 14 hours with an electrical output of 100 to 150 mW. Unwanted by-products are formed such as CO and CH4 at no more than 22 and 2 ppm and CO2 is captured as carbonate. This laboratory scale needs to be pilot tested towards production.
5.3 Hydrogen and biofuel production from biogas (biogas)
5.3.1 Biogas upgrading
Biogas is a high-energy gas fuel derived from anaerobic digestion (AD). Raw biogas containing 60% CH4 and 40% CO2 can be produced using several raw biomass feedstocks and waste. Compared to natural gas biogas is energetically inferior due to the high amount of C°2 and other contaminants in the product [43]. But compared to traditional fossil fuels, fuel produced from biogas can reduce waste management costs and provide environmentally friendly transportation fuel.
To increase energy content, biogas needs to be upgraded to reduce CO2 emissions by 75%-200% when compared to fossil fuels [44] [45]. In situ biogas upgrading involves the gas-liquid phase interaction in an anaerobic reactor being regulated in a way that results in increased methane levels in the resulting biogas. In situ upgrading methods take the form of adding certain chemicals (e.g. salt and carbon or gas sources) or by adjusting certain process parameters (i.e. digestion pressure and flow) [46] The addition of H2 to anaerobic digestion (AD) once successfully commercialized could open a new door for methane upgrading from biogas plants.
Until now biogas has typically been used in low-value applications such as heating and as a fuel in engines or even just boiler retrofits.
5.3.2 Convert biogas into synthesis gas, biomethane
An emerging strategy is to convert biogas into syngas (a mixture of H2 and CO), which can then be used to obtain liquid fuels and high-value-added chemicals. Interest also exists due to the role of bi-reforming [47] and tri-reforming in CO2 capture and utilization. New research efforts have discovered efficient and effective reforming catalysts as specifically applied to biogas [48]. The Tri-Reforming reactions of methane include 3 main reactions according to equations (8), (9) and (10).
Reforming with steam: CH4 + H2O ↔ 3 H2 + CO (8)
Dry reforming with CO: CH4 + CO2 ↔ 2 H2 + 2 CO (9)
Partial oxidation: CH4 + 1/2 O2 ↔ 2 H2 + CO (10)
With the aim of developing and testing a membrane reactor (MR) for hydrogen production from biogas, a work within the European project BIONICO evaluates the techno-economics of hydrogen production from biogas with steam reforming (SR) and automatic thermal reforming (ATR) systems [49].
Two types of biogas were tested, one generated from a landfill and one generated by an anaerobic digester. The SR system achieves a calculated maximum efficiency on LHV of 52% at 12 bar while the ATR is 28% at 18 bar. Economic analysis determined hydrogen production costs to be approximately $5/kg hydrogen for the SR case.
In the future, advanced catalysts with higher activity need to be developed to improve reactant conversion. Additionally reforming conditions for reactants with different compositions can be further optimized. Unreacted reagents such as water can be recycled to improve production economics. Finally, economic evaluation and scale-up of biogas reforming (especially tri-Reforming) should be further investigated. From syngas, biofuels can be prepared.
The H2 and biogas mixture can be processed in another reactor facilitating catalytic bioconversion to high quality biomethane (Figure 9). Power to Gas (PtG) can link the electricity and gas grids by using excess renewable electricity to produce H2. Continued conversion of biogas into stored biomethane for direct injection into existing natural gas grids provides benefits such as fossil fuel-free transportation that has improved lifecycle emissions from power generation plants and vehicles as well as support the transition to the future H2 society. In a system (PtG) using renewable energy and biogas, the following two steps are used: 1. Electrolysis and 2. Methanation (figure 9).
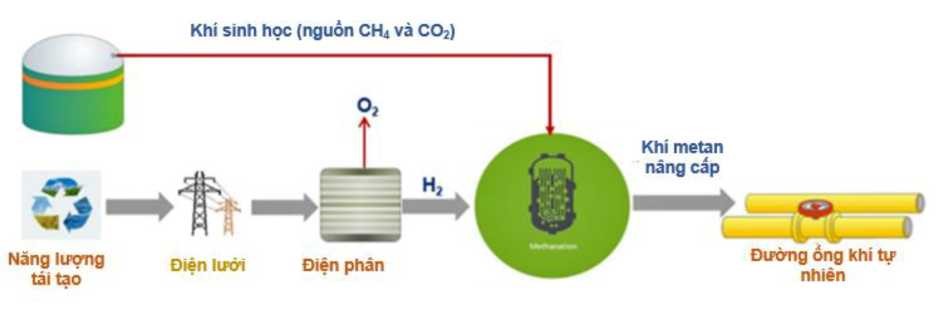
Figure 9. Biomethane production process from biogas [50]
The BioRoburplus project under the EU-funded HORIZON program will develop a pre-commercial steam reformer for sustainable and decentralized production of hydrogen from biogas without the need for primary CO2 removal. The pilot plant will supply at least 50 Nm3/h (107 kg day) of H2 with 99.9% purity and 1.5 bar with 80% efficiency. Ways to achieve this goal are i) high heat integration ii) using pressure swing adsorption (PSA) exploiting temperature to preheat animal feed iii) minimizing power consumption through CO2 removal before PSA [51].
5.4 Hydrogen production by methane pyrolysis
This is an emerging technology that is attracting attention because it has the ability to use 3-5 times less electricity to produce the same amount of hydrogen compared to water electrolysis (i.e. green hydrogen) however there are still techno-economic barriers to scale-up [52].
In this process, natural gas (methane) is heated to about 800°C in the absence of oxygen to produce carbon (black coal) and hydrogen without emitting c〇2 as in reaction equation (11). High temperatures can be achieved through conventional means (e.g. electric furnaces) or using plasma. This technology is especially suitable in locations with large natural gas volumes and very low costs, but c〇2 storage capacity is limited because it requires more natural gas than conventional methane reforming. steam.
CH 4 → 2 H 2 + C (S) (11)
Carbon (black carbon black) from pyrolysis is a by-product that can be used in other applications such as building materials or replacing coke in steelmaking, or for use in tire making or electrode...
Currently the technology readiness level (TRL from 3 to 6) is lower than that of SR, ATR and electrolysis. Different reactor technologies are at various stages of experimental and pre-commercial development as new advances. Monolith Materials (in the United States) uses thermal plasma to create the necessary high temperatures. After operating a pilot plant for four years,the company launched an industrial plant in 2020 (in Nebraska) and is planning to build a commercial-scale plant to produce ammonia from methane. To convert biogas into hydrogen and graphite, Hazer Group (Australia) is building a demonstration plant for catalytically assisted fluidized bed reactor technology. BASF (Germany) is developing an electrically heated fluidized bed reactor process. Together with RWE the company announced a project to use electricity from offshore wind to produce hydrogen from electrolysis and for a methane pyrolysis plant in 2021. Gazprom (Russia) is developing a thermal process Plasma-based methanogenesis [1].
Cost forecasts vary, some studies show a feasible lower cost of green hydrogen of 1-2.5$/kg of hydrogen (assuming a selling price of 10-150$/ton of carbon black (0.03- $0.45/kg hydrogen) and natural gas price $4/MMBtu) while other studies suggest a higher cost than green hydrogen ($1.9-2.6/kg hydrogen assuming no black carbon selling at $5.2/MMBtu). Uncertainty surrounding hydrogen costs is partly due to the expected selling price of the carbon black byproduct.
Challenges and limitations in ongoing technical issues include: I) mastering metabolic rates at scale II). Carbon blockage (remedied at laboratory scale via liquid metal reactor process) III) Low purity of hydrogen IV) Low efficiency (about 50%). There are also residual emissions from methane mining.
6 Conclusion
The trend of developing H2 technology worldwide is happening very strongly. Efforts are being focused on optimizing the water electrolysis process using renewable energy by increasing durability, increasing capacity, and reducing precious metal content in the catalyst, thereby reducing production costs. . Seawater electrolysis technology has also recently achieved a breakthrough thanks to finding an electrode material that is not corroded by chloride ions. Research directions also seek solutions to use biomass and biogas combined with excess electrical energy to produce hydrogen and synthetic fuels. An emerging technology is researching methane pyrolysis because it uses less energy. In the context that Vietnam is a country with abundant renewable raw materials such as wind, sunlight, and biomass resources in the tropics, but is currently very short of electricity and fuel, the development of hydrogen technology is extremely important. necessary. Paying attention to tracking new advances in hydrogen production technology around the world can help guide strategic research and development appropriate to the country's circumstances. Research directions and experimental implementations to utilize renewable energy such as electrolysis (especially seawater electrolysis), fuel production from biomass, and methane pyrolysis are notable beginnings to master. Hydrogen technology contributes to energy transition and environmental protection.





