Dr. Nguyen Van Nhu, Truong Nhu Tung, Dr. Dinh Van Thinh, Dr. Nguyen Viet Anh
Institute of Energy and Climate, Juelich Center for Scientific and Technical Research, Germany Email: nguyen3vannhu@yahoo.com
Summary
Climate change and fossil fuel depletion are the main reasons leading to the birth of a new energy transition strategy - Hydrogen Strategy 一 like Germany, EU, US, Japan, Korea, China. China, Australia, England, and Denmark have built and started implementing. As a very clean-burning fuel (producing only steam), hydrogen will play an important role in the energy transition to a zero-emission society c〇2. The article introduces recent advances in environmentally friendly hydrogen application technology in transportation, industry and power generation around the world. Progress in fuel cell research and development is the core technology that is emphasized. The article also presents hydrogen technology and safety challenges and barriers that need to be overcome. Based on the advances and challenges as well as barriers of social acceptance, some suggestions are proposed to promote Hydrogen technology and environmentally friendly smart energy systems for the long-term benefit of people. proposed people.
Keywords: Hydrogen applications, fuel cells, transportation, steel, cement, power generation, hydrogen safety,
1 General introduction
Hydrogen is considered a key element in the world's current energy conversion. Hydrogen can be used directly in pure form or as a basis for the synthesis of liquid or gaseous hydrogen fuels such as synthetic methane or synthetic diesel as well as for other energy carriers such as ammonia. To date, most hydrogen is currently used in the industrial sector mainly in oil refineries and ammonia production and to a smaller extent for the production of methanol and other chemicals as well as steel production [first].
In the residential sector, hydrogen is used in fuel cell-based applications called combined heating and power (CHP) systems. Proton electrolysis membrane (PEMFC) and solid oxide fuel cell (SOFC) technologies are most commonly used. Both fuel cells in the CHP can be controlled thermally or electrically and can be deployed as small or micro CHPs due to their compact size. The fuel for PEMFC is direct pure hydrogen, the fuel for SOFC can use hydrogen or natural gas or biogas or a mixture thereof, where the conversion to hydrogen takes place inside the device. If the heat generated is of high enough temperature, this system can also provide cooling through adsorption.
In the transportation sector hydrogen is especially important in reducing c〇2 emissions and providing large amounts of energy. Fuel cell vehicles (FCEVs) such as buses, long-haul passenger cars and trains, as well as certain ships, are major future applications of hydrogen.
Hydrogen can be used to produce electricity because it can be converted into electricity by combustion or fuel cells. Direct hydrogen combustion can take place in internal combustion engines (in most cars and turbines, for example). Fuel cell-based electricity generation is mainly deployed as a system that supplies continuous power to the grid system. Their main function is to provide backup power to critical processes such as hospital communications infrastructure and the like in the event of power outages and in remote locations. Their advantages are reliable durability and environmental friendliness.
This article presents some of the latest advances in environmentally friendly hydrogen application technology for the transportation, industrial, and power generation sectors. The article also presents the challenges and barriers in terms of technology, hydrogen safety and social acceptance. Based on these advances and challenges, the article also proposes some suggestions in hydrogen technology in Vietnam.
2 Applications of hydrogen and hydrogen-based fuels for transportation
The process of reducing CO2 emissions in transportation is one of the biggest challenges in responding to climate change globally. Global transportation is responsible for approximately 23% of global emissions from the combustion of fossil energy hydrocarbons in road, maritime and aviation vehicles. Reducing emissions in this sector is particularly difficult because one of the key requirements for an energy source is mobility, and hydrocarbons are among the highest energy density substances available. On the other hand, the public health burden due to pollutants such as NOx and SOx gases emitted from vehicles is very large, so cleaner energy sources are needed [2].
To solve this problem, efforts have focused on hydrogen-powered batteries and fuel cells along with improvements in efficiency (hybrid vehicle development and optimization) and conversion. fuel, for example using biofuel or natural gas instead of gasoline.
Figure 1 below compares greenhouse gas emissions from fuel cell vehicles and traditional vehicles using internal combustion engines. It can be seen that for fuel cell vehicles, greenhouse gas emissions are lower, about 225 gc〇2/km if hydrogen is produced by natural gas reforming and can be reduced to 125 gc〇2/km. gc〇2/km if hydrogen is produced by electrolysis using wind power. Meanwhile, with vehicles using traditional internal combustion engines, each kilometer of journey will emit a higher amount of CO2, at nearly 250 gc〇2/km.
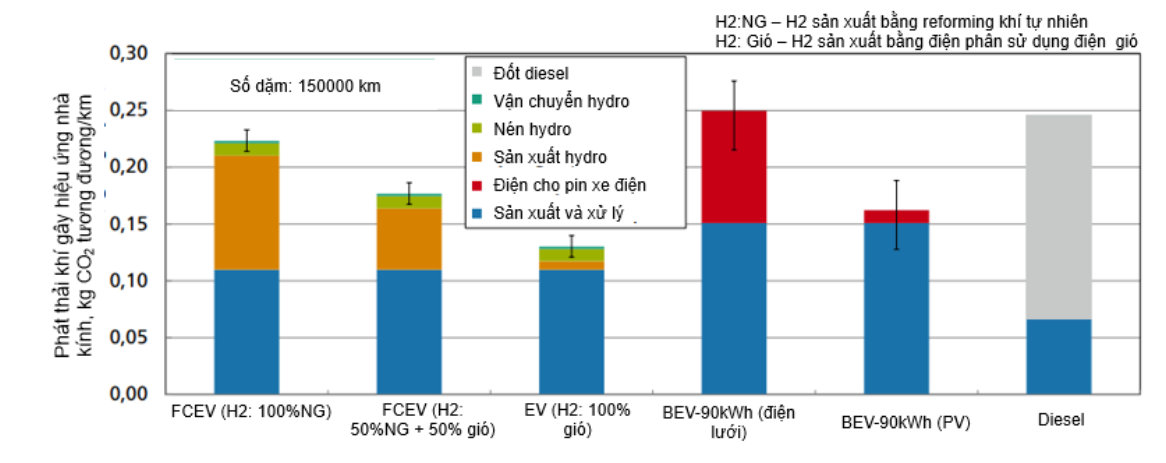
Figure Greenhouse gas emissions from traffic activities in the period 2020-2030 [Source: Fraunhofer, Germany ISE Solar Energy Systems)
2.1 Fuel cells for transportation
Although in recent times, electric batteries have been the dominant technology due to their advantages in meeting production costs per vehicle and the availability of supporting infrastructure, fuel cells can provide long-term substitution advantages. The most obvious advantage of fuel cells is their high energy density suitable for large-scale transportation, long-distance transportation, and short refueling time. The bigger advantage of switching to a hydrogen-fueled transportation system is that a “storage source” from a renewable energy source provides intermittent (fluctuating over time) electricity in the form of a stable chemical. . According to experts, hydrogen is the only energy carrier that has the potential to replace fossil fuels in road transport in the long term.
A fuel cell has a simple structure consisting of three layers located next to each other. The first layer is the fuel electrode (anode), the second layer is the ion-conducting electrolyte, and the third layer is the oxygen electrode (cathode) shown in figure 12.
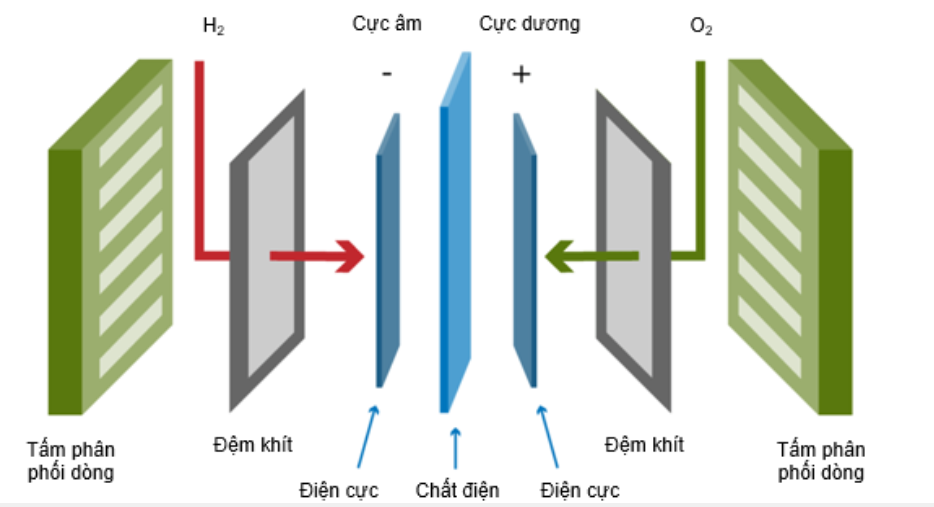
Figure 221. Structure diagram of fuel cell [3]
Chemically, fuel cells are the reverse reaction of electrolysis. Fuel cells operate on the principle: fuel and air (oxygen) are physically separated by an insulating electrolyte. The half-reactions take place at the electrodes on either side of the electrolyte, ion transport occurs across the electrolyte. The released electrons travel from the anode through the external circuit to the cathode and create usable electrical energy.
Equation (16) represents the overall chemical reaction occurring in a fuel cell using hydrogen:
2 H2 + O2 → 2 H2O
Because an individual battery cell can only create a very low voltage, depending on the voltage needed, many batteries are combined together, that is, overlapped in the necessary quantity. People often call such overlapping of battery cells a stack.
The two most commonly used types of fuel cells for transportation are proton exchange membrane fuel cells (PEMFC) and solid oxide fuel cells (SOFC).
PEMFC fuel cells with a polymer membrane electrolyte must be saturated with water so that solvated protons can move in the electrolyte but do not allow electrons to pass through. The combination of water and sulfonic acid is essential to allow protons to enter the membrane easily. PEMFCs are the most commercialized type today due to their low operating temperature (50-100°C), short start-up time, and ease of use of an oxidizer (atmospheric air). These characteristics make PEMFC ideal for mobile transportation solutions. The disadvantage of PEMFC is that it requires Pt catalyst in the electrode material and is easily damaged when exposed to CO.
SOFC fuel cells with a solid oxide electrolyte usually zirconia stabilized by yttria (YSZ). These materials typically have suitable ionic conductivities in the range of 650-1000 °C. SOFCs have extremely attractive properties for use in transportation in that they do not require expensive and rare platinum group metals in the electrode material. In particular, SOFC has the ability to use not only hydrogen but also hydrocarbon fuels with high efficiency, and can withstand impurities in the fuel. Even when using hydrocarbon fuels, most modern SOFCs have a system efficiency of 50% and can reach targets of 65% or more. This is higher than the PEMFC efficiency (typically around 36-4540%) using hydrogen [2].
A typical example of the use of fuels other than hydrogen in SOFCs is the 2016 Nissan launch of the world's first solid oxide fuel cell vehicle powered by Bio-Ethanol fuel with a range of 600 km [4] .
With a bioethanol system, c〇2 emissions are neutralized from photosynthesis, forming a biofuel that allows it to have a carbon neutral cycle with virtually no overall c〇2 increase.
In the transportation sector, fuel cell electric vehicles (FCEVs) have a decisive advantage over battery electric vehicle (BEV) options as they are suitable for large-scale transportation, large distance transportation, high power density, short refueling time and smaller volume.
The purpose of fuel cells for transportation is to provide propulsion to vehicles directly or indirectly. The following application areas are being developed:(1)forklifts, light vehicles (LDVs), (2) buses and trucks, (3) trains and trams, (4) ferries, Cargo ships and small boats, (5) manned light aircraft, (6) unmanned aerial vehicles (UAVs), (7) unmanned undersea submarines (UUVs).
The powertrain system diagram in FCEV is simulated in Figure 23.
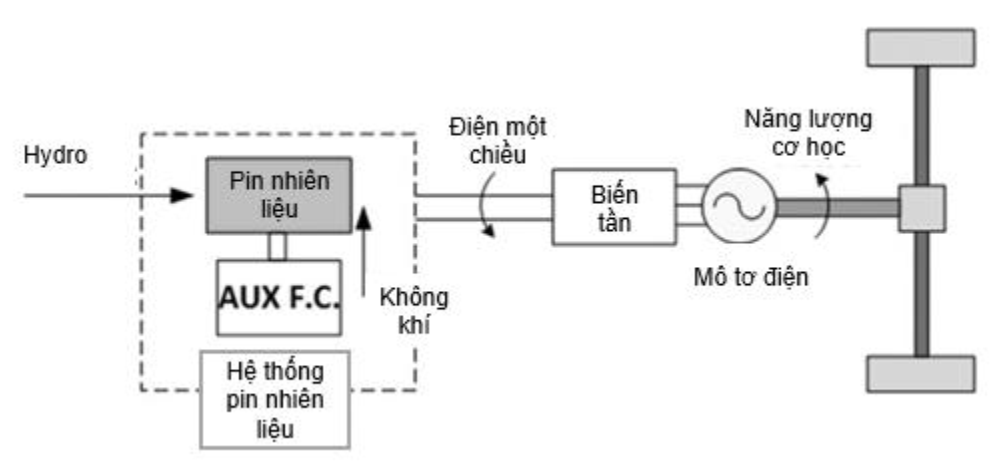
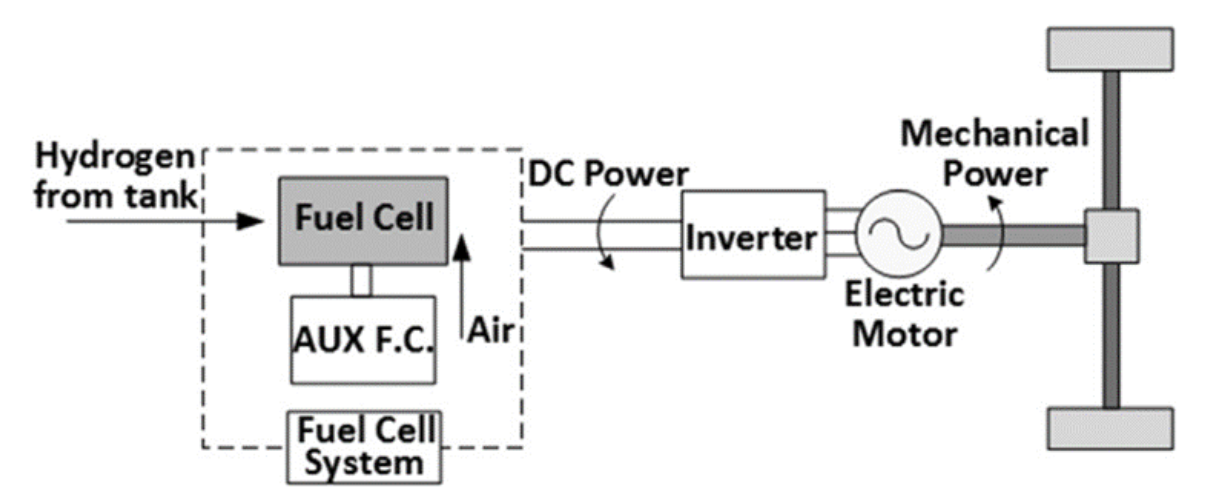 Figure 332. Diagram of the powertrain system in a fuel cell car (source from public information)
Figure 332. Diagram of the powertrain system in a fuel cell car (source from public information)
The energy efficiency of FCEV is shown in figure 43.
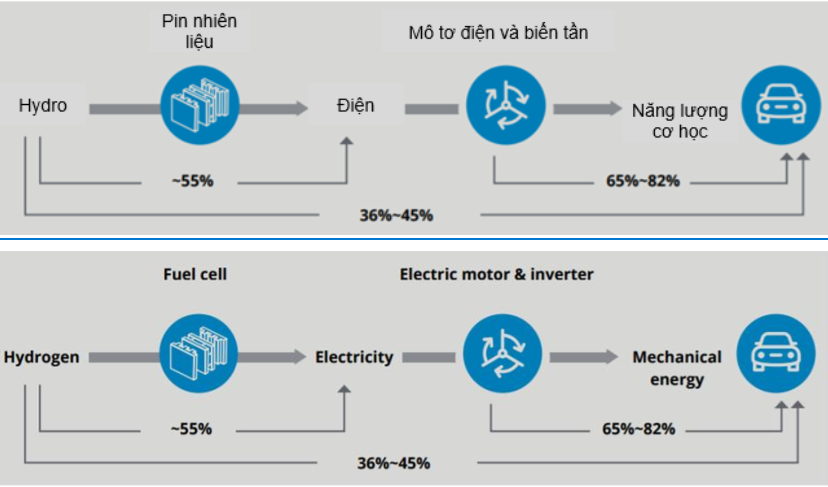
Figure 34. Energy efficiency of FCEV
SOFC may be a viable technology with advances in performance expected over the next few years. From the anode side, improvements in catalysis as well as carbon and sulfur tolerance are needed. On the cathode side, computational material design appears to become more important as materials become more complex with an increasing number of particles and different structures that require changing the nanoscale of the surface. electrode surface. In electrolytes, using new materials in suitable electrolytes at lower temperatures will allow for increased use of SOFCs in transportation [2].
The most recent advances in direct ammonia fuel cells based on the principle of SOFC fuel cells were presented by Jeerh et al [5]. The authors have compared the pros and cons of different direct ammonia fuel cells (fig. 54) based on operating principles and have demonstrated how close this type of technology is to integration with future applications including the transportation sector. Currently, several challenges such as material selection, NOx conversion, low power density and durability still have to be overcome.
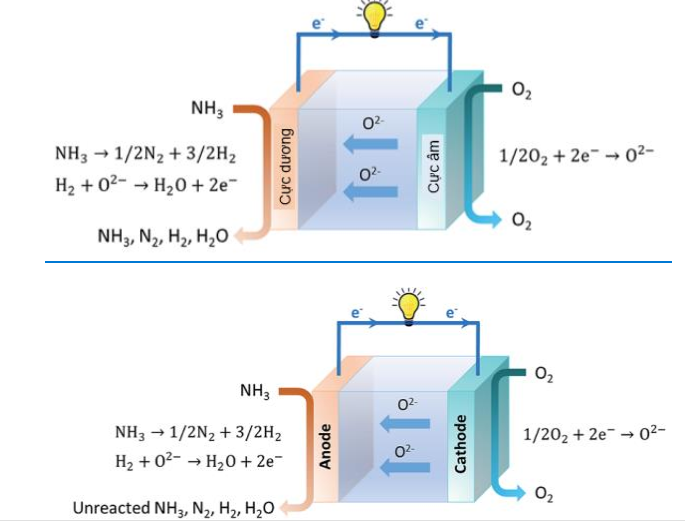
Figure 45. Schematic illustration of a SOFC fuel cell using ammonia directly [50][5]
An important application of fuel cells, especially SOFCs, is in APUs.
One of the earliest full-scale trials completed in 2010 was of an auxiliary power unit (APU) on a truck where the APU supplied power to on-board services during an overnight stop. A similar system was developed and installed by AVL (Austria) on a Volvo truck with similar results [6].
Larger systems have also been built. Thyssen Krupp and Sunfire are developing an oil-fired 50 kW SOFC. Construction of the 50 kW device demonstrator began at the end of 2015 at the thyssenkrupp Marine Systems shipyard in Kiel. In 2016, the testing phase was able to demonstrate an overall system electrical efficiency of 55% and fuel utilization of 73% [7].
In addition to hydrogen application in FCEVs, a research direction on hydrogen application in internal combustion engines is also being implemented. KEYOU, a German company, is researching the development of a hydrogen internal combustion engine with appropriate technological methods including efficient gas fuel injection valves and exhaust gas recirculation without major changes to the combustion engine. in basic. Compared to conventional fuels , hydrogen has the highest energy content. In the combustion process developed by KEYOU, hydrogen burns with oxygen in atmospheric air to form water without CO2 emissions [8].
2.2 Road traffic
The total CO2 emissions of different car models are shown in Figure 56. Car models using combustion engines have more CO2 emissions than FC fuel cell cars. (Toyota) [9]
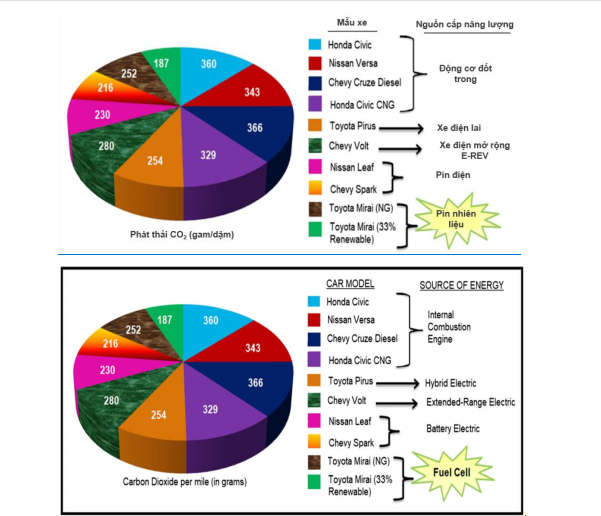
Figure 56. Comparison of CO2 emissions from different car models [10]
The PEMFC fuel cell has reached a state of technological readiness as several major auto companies are commercially leasing and selling fuel cell electric vehicles (FCEVs) including Toyota, Honda and Hyundai that can drive long distances. Maximum distance from 500 to 600 km for 1 refueling. These FCEVs claim superior vehicle speed and driving range and durability compared to conventional internal combustion engines (ICEs) and in most cases are better than battery electric vehicles (BEVs).
The remaining challenges and major improvements to PEMFCs that need to be addressed are performance, durability, and cost at high current densities. These issues are expected to be resolved in the next decade, during which time hydrogen infrastructure should be widely deployed [11].
The cost of automotive fuel cells has dropped 70% since 2008 thanks to technological advances and growing sales of fuel cell electric vehicles (FCEVs). Thanks to the efforts of South Korea, the US, China and Japan, the number of FCEVs on the road increased more than sixfold from 7 000 in 2017 to more than 43 000 by mid-2021. In 2017, practically all FCEVs are all passenger cars. Today, one in five are buses and trucks showing a shift to the long-haul segment, where hydrogen can compete better with electric vehicles. However, the total number of FCEVs is still much lower than the estimated 11 million BEVs on the road today.
Compared to BEVs, heavy-duty vehicles (150-400 kW) FCEVs have a significant advantage because of the scalability of fuel cells in both capacity and energy by increasing the size and increasing the number of battery stacks. fuel (stack) or hydrogen tank with much less additional weight than a lithium-ion battery. Commercial deployment of heavy vehicles such as trucks requires less infrastructure investment because fewer refueling stations are needed due to designated dedicated routes. However, the drive cycles and operating conditions vary among class vehicles
Their heavy weight as well as their significantly longer lifespan require significant improvements in durability and a greater focus on fuel efficiency compared to light vehicles [12].
According to data collected by Samsun et al., there were 34,804 fuel cell vehicles (FCEVs) of all types in operation worldwide as of the end of 2020. This total includes passenger cars (up to 9 seats), buses, light commercial vehicles up to 3.5 tons, medium trucks and heavy trucks [13].
More than 40 000 FCEVs were available globally at the end of June 2021. Supply increased by an average of 70% annually from 2017 to 2020, with growth only 40% in 2020 due to the Covid-19 pandemic [ first]. Global FCEV deployment is largely focused on light passenger vehicles (PLDVs), which accounted for 74% of registered FCEVs in 2020. Three commercial fuel cell PLDV models are being marketed (Hyundai NEX〇,Honda Clarity and Toyota Mirai second generation). Buses, despite being deployed earlier and offering a larger number of fuel cell models (12 according to Calstarts Zero Emissions Technology Inventory tool) currently make up only 16 of the total FCEV stock . Nearly 95% of FCEVs in China are fuel cell trucks with more than 3,100 in operation in 2020.
The most vehicles are in Korea, followed by the US, China and Japan. This year, South Korea took the lead for the first time, replacing the US. The distribution on the continent shows 65% of vehicles in Asia, followed by 27% in North America and 8% in Europe. The vehicle structure is mainly passenger cars (74.5%), followed by buses (16.2%) and medium-duty trucks (9.1%). FC forklifts are in the commercial stage especially in the US with 25,000 units [13].
Daimler Truck AG and Volvo Group have announced a joint venture center to develop the production and commercialization of fuel cell systems for long-distance transport. Together with IVECO OMV and Shell, both companies have also signed the H2Accelerate agreement to collaborate on large-scale hydrogen truck deployment in Europe. For a full overview of the goals supporting electric vehicle development, see the IEA Global EV Outlook 2021.
Ceres Power and Weichai Power 2020 collaborate to develop a bus system for China using SOFC fuel cell technology and compressed natural gas fuel. It is expected that 2022 will be born. Importantly, the deal with Weichai Power includes a potential joint venture to establish a manufacturing plant in China, highlighting the potential of SOFCs for transportation applications to go into production [14] [15].
Hydrogen refueling station (HRS) infrastructure worldwide is currently developing more slowly than the pace of FCEV development. The number of hydrogen refueling stations (HRS) increased by an average annual rate of nearly 20% between 2017 and 2020. The ratio of FCEVs to、HRS numbers is increasing, especially in countries with the highest FCEV sales. In 2020, the ratio reached almost 200:1 in South Korea and 150:1 in the United States compared to just 30:1 in Japan. At the end of 2020, 540 HRS were in operation including both public and private installations. A continent-based analysis shows that most HRS are concentrated in Asia with a total of 278, followed by Europe with 190 and 68 in North America. The country with the highest number of stations is Japan (137). Germany (90) and China (85) are ranked second and third, respectively, in this ranking [13]. Station refueling pressure varies depending on the vehicle market served. In most countries, the majority of hydrogen dispensing stations are at 700 bar to serve fuel cell cars. In China, most distribution stations serving bus and truck fleets have a pressure of 350 bar [1].
2.3 Railway traffic
Hydrogen and fuel cell technology has been proven in rail applications. In 2018, the first commercial service of a hydrogen fuel cell passenger train (developed by Alstom) started on a 100 km route in Germany. Two Alstom trains in Germany have run 180 000 km and many other countries have begun testing and using fuel cell trains.
Where direct electrification of power lines for rail systems is difficult or too costly, implementing fuel cell rail applications could help reduce CO2 emissions in this sector.
In 2020, a hydrogen train entered regular passenger service in Austria and trials have begun in the UK and the Netherlands. In Europe, France, Italy and the UK have all placed orders for hydrogen fuel cell trains while the largest fleet of 27 hydrogen trains is expected to begin permanent regular operation in Germany in 2022 [ first].
Countries such as China, South Korea, Japan, Canada and the United States are also expressing interest in hydrogen fuel cell trains. In addition to passenger trains, hydrogen tram lines and switched locomotives are in various stages of development and deployment.
2.4 Waterway transportation
The maritime industry today emits about 2.5% of global carbon emissions equivalent to 940 million tons per year [16].
Commercial operations of the fuel cell ferry are expected to begin in 2021 in the United States and Norway. Most of the hydrogen-fueled vessels currently being demonstrated or planned to be deployed in the next few years are passenger and tugboats typically with fuel cell capacities ranging from 600 kW to 3 MW. Furthermore, a recent EU partnership aims to build a hydrogen ferry with 23 MW of fuel cell power [1].
Further information on fuel cell applications in the marine sector can be found in reference to van Biert et al. [17]
Besides hydrogen fuel cell application for small vehicles, fuel cells using ammonia directly also have some applications [5]
Although FC ammonia has performance advantages, large-scale development and deployment will take longer than using ammonia in ICE internal combustion engines [18].
In addition, the trend of researching and applying NH3 as fuel for large internal combustion engines running at sea is attracting special attention. Green ammonia (produced from renewable energy) can be used in internal combustion engines to eliminate c〇2 emissions of ships [19]
The overall reaction of ammonia combustion is [20]:
4 NH3 + 3 O2 → 2 N2 + 6 H2O
The efficiency of pure ammonia combustion is still low, but mixing ammonia with other fuels (e.g., hydrogen) can help overcome the unfavorable combustible properties and improve efficiency [18] [21][ 22]. Dual-fuel (ammonia-hydrogen) combustion engines will be the most promising route for ammonia to enter the marine sector.
Ammonia is a carbon-free energy carrier but combustion emissions can be harmful to the environment if they remain untreated. NOx emissions can be removed through conventional exhaust gas treatment processes. N2O emissions from ammonia combustion are a major concern. Stringent regulations on N2O emissions need to be established to ensure that ammonia engines are compatible with the long-term goal of decarbonizing shipping. Therefore,,N2〇 can be integrated into carbon pricing policies or restricted through emission standards. Due to the risk characteristics of NO2, its use may not be applicable in all segments of the maritime sector, e.g. passenger ships.
Major industry stakeholders have announced plans to produce 100 ammonia-fueled marine engines as early as 2023 and offer ammonia retrofit packages for existing vessels from 2025. Methanol also has proven to be a fuel for the marine sector and is relatively longer lasting than hydrogen and ammonia. With its compatibility with existing marine engines, methanol may be a short-term solution to reducing shipping emissions but ultimately ammonia offers greater decarbonization potential.
2.5 Air traffic
Planes flying on standard jet fuel (kerosene) emit 3.15 kg CO24fene per kg of fuel. This equates to about 360 tons of carbon for a 10-hour trip on a Boeing 747, and the sector as a whole emits about 3% of global carbon emissions or about 0.75 million tons per year. [16]. The International Council on Clean Transportation (ICCT) has developed a bottom-up global aviation inventory to better understand c〇2 emissions from commercial air travel. .Accordingly, passenger transportation caused about 85% of c〇2 emissions in commercial aviation. In 2019, this number reached 785 million tons (Mt) c〇2. From 2013 to 2019, c〇2 emissions related to passenger transport increased by 33% [16].
In the future, more electrical configurations will appear for aircraft. Conventional aircraft auxiliary components are known to create air pollution and noise. By using fuel cells as the APU source operating on air vehicles, air and noise pollution are reduced [23].
Unlike the existing auxiliary power unit (APU) there is a solid oxide fuel cell power unit that can operate throughout the flight to maximize fuel economy.
The expected benefits of applying a fuel cell system are (a) low emissions - significant reduction of NOx on the ground and in flight (b) high efficiency (c) fuel economy - up to 75% reduction % fuel on the ground and 30% fuel reduction in flight and (d) noise reduction - great potential for significant noise reduction on the ground.
Interest in using hydrogen for aviation is also growing. Industrial group ATAG sees a role for hydrogen fuel cells for flights up to 1600 km and hydrogen combustion for short flights and potentially for medium-haul flights. Assuming the technology is successfully developed, hydrogen fuel cells could be used in 75% of commercial flights but only account for 30% of aviation fuel. Technically, hydrogen combustion could be used for longer flights, potentially covering nearly 95% of the flight and 55% of the fuel burn, but would require equipment to minimize emissions NOx. Sustainably reduced aviation fuels including hydrogen-based fuels and biofuels will be needed to decarbonize for the duration of at least long-haul flights although means may be needed to minimize the impact of a CO2-free climate [1].
Whether used with fuel cells or burned directly with hydrogen will require new aircraft system designs. Airbus is exploring various hydrogen aircraft concepts, focusing on a capacity of up to 200 passengers and a range of 3,700 km with the goal of having a commercial aircraft by 2035. Smaller companies are investigating Hydrogen aircraft solutions include ZeroAvia which targets the first commercial offering of a hydrogen aircraft with a range of 900 km by 2024 and Universal Hydrogen which aims to develop hydrogen storage solutions and converters for the aircraft. commercial flight.
Boeing recently partnered with the Australian Commonwealth Scientific and Industrial Research Organization (CSIRO) to publish a hydrogen roadmap in aviation that examines opportunities for hydrogen use in aircraft and other airport applications (buses providing ground support equipment such as taxis and cargo trucks. Remaining technical challenges include lightweight cryogenic tanks (with minimal boiling) and development of hydrogen supply infrastructure (either near or on-site liquefaction pipelines) and high-volume liquid refueling [1].
2.6 Submarine applications
Fuel cells require oxygen to function. However, the amount of oxygen needed is eight times heavier than hydrogen. If oxygen is contained in a submarine, the ship's tonnage is too large and its buoyancy cannot be adjusted (when it sinks and when it floats). Recently, the Norwegian military technical institute FFI has successfully tested using H2O2 as an oxygen source for self-driving submarines when using fuel cells [24]
H2O2 is rated highest in terms of depth independence. This is because H2O2 is a liquid and can therefore be stored in flexible plastic bags that can withstand the pressure of the surrounding seawater. Inside the submarine there is a reaction device to create oxygen from H2O2 according to equation (3)
H2O2(l) → H2O(l) + 0.5 O2 (g)
This is an important advance, a lightweight oxygen storage solution that is not dependent on depth because the H2O2 is outside the ship's hull. The supply of pure oxygen makes the fuel cell more efficient. Fuel cells need a more compact design to accommodate the lower oxygen flow rate compared to air. Because normally air is used, the amount of oxygen is only 20%. Both factors (smaller fuel cell and smaller oxygen flow rate) contribute to the higher total energy density.
Conclusion: Fuel cells and hydrogen have great potential to advance the future of transportation. Regions including the United States, China, Europe and Japan, among others, are recognizing this trend and focusing policy efforts on developing fuel cell technology supply chains and infrastructure. Multi-faceted upper floor. Due to characteristics such as fast refueling similar to internal combustion engine vehicles (ICEVs), high energy density (i.e. lower weight than BEVs), FCEVs are an attractive solution for trucks. heavy duty and commercial vehicles. It is estimated that the total cost of ownership (TCO) of FCEVs will decrease by nearly 50% in the next 10 years due to a number of factors such as falling fuel cell system prices, the price of producing hydrogen from energy. renewables decrease as does the development of hydrogen infrastructure. Finally, FCEVs demonstrate the lowest greenhouse emissions compared to BEVs and ICEVs, and show the highest potential for improving the atmosphere due to increased use of renewable energy in hydrogen production.
3 Advances in hydrogen applications in industry
3.1 "Green" steel production technology in Europe
In Europe, about 170 million tons of crude steel are produced each year [25]. Each ton of steel produced will emit about 1.85 tons into the environment [26], the steel manufacturing industry has contributed 4% of total c〇2 emissions in Europe and accounts for 22% of industrial emissions. [27].
Steel is mainly produced through two routes: using Blast Furnace - Oxygen Blowing Furnace (BF-BOF) and using Electric Arc Furnace (EAF), of which the BF-BOF method accounts for 60% of the production technology. steel production in Europe. Among the solutions to reduce c〇2 emissions in the steel manufacturing industry, CCS technology is applied to capture carbon at several source points in the production process such as blast furnaces, coke ovens and effectively reduce emissions. The highest emissions only reach about 80% [26]. Compared to using CCS, direct reduction technology using green hydrogen and combined with an electric arc furnace is more effective in reducing CO2 emissions. The application of hydrogen in the steel production industry is approached as a reducing agent instead of carbon. When hydrogen is used as a reducing agent, the gaseous product of the reduction of iron oxide to iron is water vapor instead of CO2 gas when using coal as a reducing agent. This has contributed to reducing CO2 emissions during the steel production process. Hydrogen can be applied in the steel production industry in two ways as follows:
- Use hydrogen as auxiliary reducing agent in BF-BOF or also known as H2-BF;
- Use hydrogen as the sole reducing agent in direct reduction, called H2-DRI.
3.1.1 H2-BF 一 reduces CO2 emissions in the steel production industry
The steel production process following the BF-BOF path is shown in Figure 76.
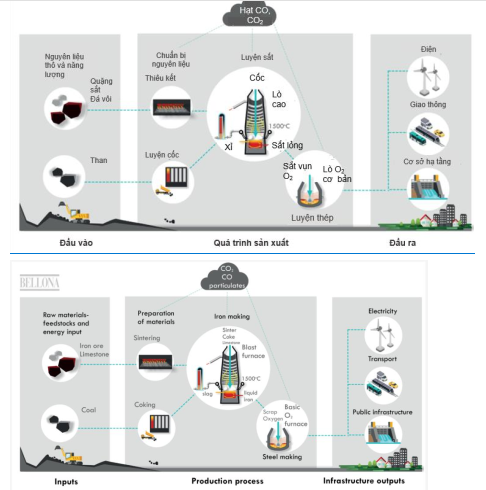
Figure 743. Steel production according to the BF-BOF route [27]
CO2 emissions during steel production in the BF-BOF pathway come from BF blast furnaces and coke ovens. The coke oven's role is to produce coke to provide heat and act as a reducing agent in the blast furnace. Using hydrogen as a fuel and reducing agent contributes to reducing CO2 emissions. The reaction of reducing iron oxide to iron using reducing agents hydrogen and charcoal is shown in the following reactions.
| Fe2O3 | + | 3H2 | → | 2Fe | + | 3H2O | (100kJ) | (4) |
| Fe2O3 | + | 3CO | → | 2Fe | + | 3CO2 | (-23.5 kJ) | (5) |
The reduction reaction with the reducing agent CO is exothermic while the reaction with the reducing agent H2 is endothermic. Besides emitting H2O instead of CO2, using hydrogen in steel production has the disadvantage of requiring higher energy consumption. For a number of technical reasons, hydrogen cannot completely replace coal, so H2-BF technology is often considered a transition step towards H2-DRI technology.
In Europe, there are a number of factories expected to use H2-BF technology in the steel production process [27]. There are plans to use hydrogen produced from water electrolysis using renewable energy electricity, and there are also some other companies that plan to use gray hydrogen while waiting for green hydrogen to truly become available at a price. both and quantity. Depending on the source of hydrogen used, the effectiveness of reducing CO2 emissions will be different. When using green hydrogen, the CO2 emission reduction efficiency is highest, reaching 21% [27] (about 1,063 tons of CO2 emission per ton of hot metal produced), while the CO2 emission reduction efficiency when using The use of gray hydrogen decreased 10 times, or 2.1%. In the case of combined use of CCS technology, that is, blue hydrogen production, the CO2 emission reduction effect can be similar to that of using green hydrogen.
Thus, the most optimal condition to reduce CO2 emissions with H2-BF technology is to use green hydrogen produced from electrolysis using renewable energy electricity. However, as mentioned above, hydrogen cannot replace all coal, so the steel produced in this case cannot yet be called "green" steel.
3.1.2 DR-EAF 一 reduce CO2 emissions in the steel production industry
For the DR-EAF technology, hydrogen is used as a reducing agent that reduces iron ore to a solid state (called direct reduction DIR), creating sponge iron. The sponge iron is then fed into an Electric Arc Furnace, which uses electrodes to create an electric current that melts the sponge iron and produces steel. This steelmaking process still requires a certain amount of carbon, which can come from coal powder, biomethane or other biological carbon sources. For this reason, if the process uses all green hydrogen and renewable energy electricity, it will still emit about 53 kg of CO2 per ton of steel produced [28].
Figure 78 below shows the DR-EAF steelmaking process using a shaft furnace or a fluidized bed reactor with green hydrogen as the sole reducing agent.
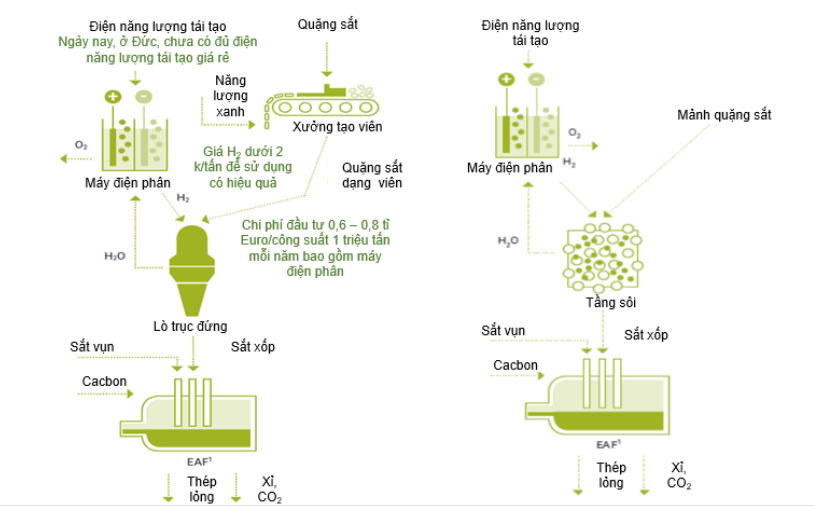
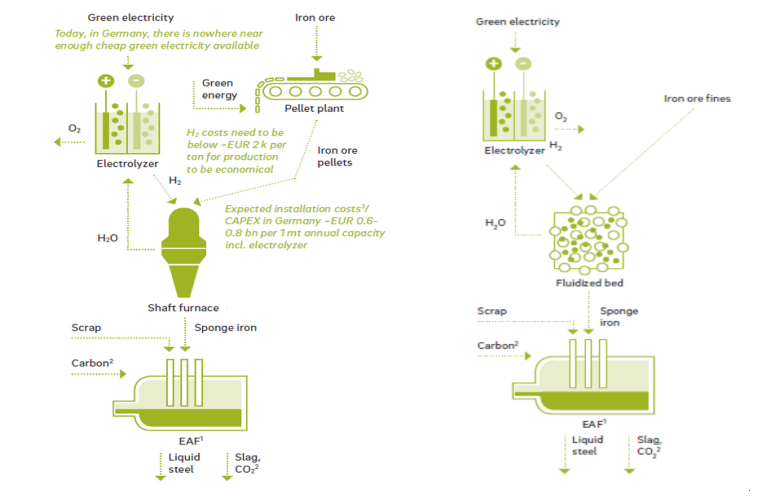 Figure 78. Steel production process according to the DR-EAF route [28]
Figure 78. Steel production process according to the DR-EAF route [28]
Direct iron reduction technology is not a new technology, it has been commercialized since the late 1960s, but does not use pure hydrogen. In Europe, there are several DIR projects in various stages, from planning to pilot operations. The majority will use DIR technology combined with the EAF pathway, the remainder will use a combination of DIR-BF-BOF. Due to the unprepared quantity and price of green hydrogen, the use of green hydrogen in projects is still very limited [28].
Depending on the reducing agent used and the DIR combination plan, the effectiveness of reducing c〇2 emissions will be different, in which [28]:
- DRI-EAF using natural gas will emit 0.95 tons of CO2 per ton of steel;
- DIR-EAF uses hydrogen from water electrolysis using grid electricity, emitting 0.175 tons of CO2 per ton of steel;
- DIR-EAF uses green hydrogen for very high emission reduction efficiency, up to 95%. With an expected DIR capacity in Europe of 20.45 million tons/year, it is necessary to use 66 TWh of electrical energy per year, 'accounting for about 28% of renewable energy capacity in Germany in 2019.
Thus, it can be seen that DIR-EAF technology using green hydrogen contributes to reducing CO2 emissions very effectively. However, with the current limited share of renewable energy, steel production using DIR with green hydrogen is unlikely to account for 100% of Europe's steel production by 2050.
3.2 Hydrogen application in cement production industry
Cement production in the world has increased gradually over the years, from 3.27 billion tons in 2010 to 4.1 billion tons in 2020, of which cement production in European countries is 0.2 billion tons [ 29]. Cement production is one of the sources of large CO2 emissions in industry, accounting for 7%, after steel production industry. On average, 1 ton of cement produced will emit about 0.9 tons of CO2 [30]. Thus, in 2020, the cement production industry worldwide contributed about 3.7 billion tons of CO2 emissions, of which 0.18 billion tons of CO2 emissions came from Europe. Besides, the cement manufacturing industry has the highest CO2 emissions per unit of revenue, up to 6.9 kg/USD of revenue, 5 times higher than the steel manufacturing industry [30]. .
CO2 emissions in the cement production industry come from most stages, the cement production process presented in Figure 98 below clearly shows this. Emissions mainly come from the process of burning fuel to provide energy and most come from the calcination of limestone that occurs according to the reaction below. The CO2 emission ratio between these two processes is 40:60.
| CaCO3 | → | High | + | CO2 | (6) |
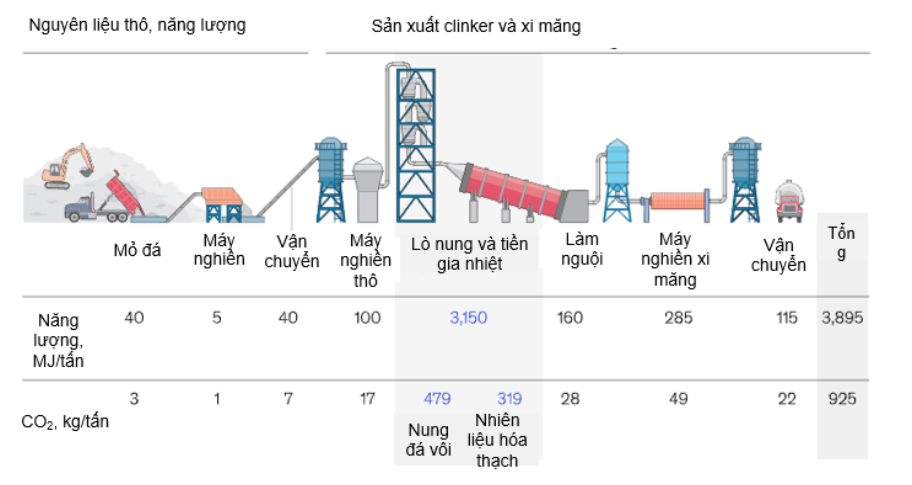
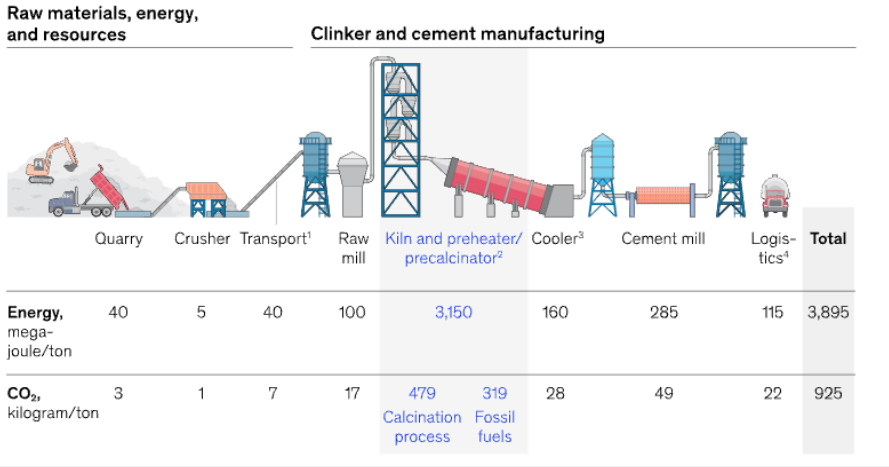 Figure 98. Cement production process [30]
Figure 98. Cement production process [30]
According to Mckinsey's forecast, by 2050, the cement manufacturing industry can reduce emissions by 75% compared to 2017. Of which, only a small part will come from operational advances such as using more economically. saving energy, replacing clinker, most emissions can be reduced through technological advances such as carbon capture, utilization and storage technology [31].
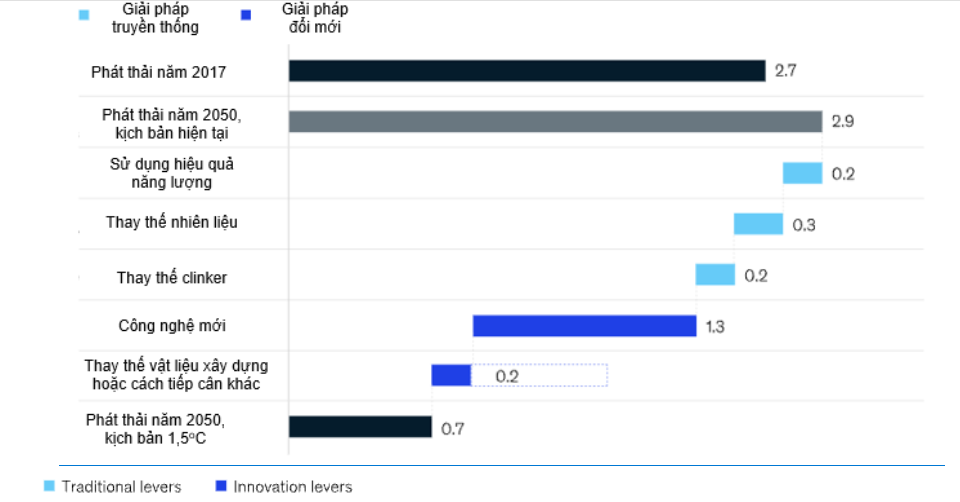
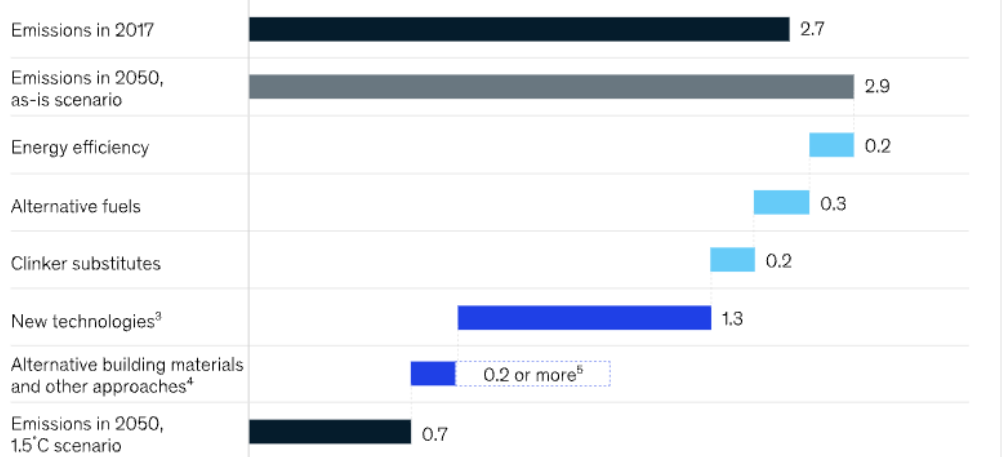
Figure 910. Method to reduce CO2 emissions in the cement production industry [31]
Some methods to reduce CO2 emissions in the cement production industry include:
- Converting cement kiln from wet to dry kiln. Clinker production in modern dry kilns reduces energy consumption by 85% compared to wet kilns, replacing all wet kilns with dry kilns with modern advanced technology [32], which can improve emissions by 10%. by 2050;
- Replacing clinker: Clinker is the basic component of cement. According to the IEA and CSI Roadmap, the current average clinker content of 0.65 needs to be reduced to 0.6 to meet the average temperature target. Global average increase at 2oC according to the Paris agreement. Clinker replacement materials such as blast furnace slag, fly ash or limestone are effective in reducing CO2 emissions by about 10% as clinker replacement;
- Applying CCS technology: applying advanced technologies plays a key role in reducing CO2 emissions for the cement production industry [34].
Besides, it is estimated that 10% of CO2 emissions in the cement industry come from transportation and the electrical energy needed to operate machinery and equipment. A standard cement plant with a capacity of 3,000 tons per day will consume 20 to 25 MW of electricity [33]. Replacing traditional fuels used in cement production with green hydrogen or renewable electricity contributes to a 15% improvement in emissions by 2050.
3.3 Hydrogen application in electricity production
According to the International Energy Agency (IEA), the power sector is the third largest source of c〇2 emissions in the world. About 64.5% of electricity is produced through burning fossil fuels. c〇2 emissions in electricity production come from the process of burning coal or natural gas to operate boilers (supplying steam for steam turbines) or gas turbines. Besides c〇2 emissions, the electricity production process also emits acid gases such as SOx and NOx, which have negative impacts on air quality and human life.
Hydrogen can contribute to reducing c〇2 emissions in the energy sector by (1)acting as an energy storage system with electrolyzers and fuel cells; (2) Directly replace fossil fuels in the electricity production process. Hydrogen and fuels derived from it such as NH3 can directly replace natural gas in gas-fired power plants or use NH3 as a substitute for coal in coal-fired power plants. Using hydrogen instead of fossil fuels not only contributes to reducing CO2 emissions but also completely eliminates other impurities in power plant exhaust fumes such as SOx, volatile hydrocarbons, mercury... However , it is necessary to pay attention to the problem of NOx in exhaust gas when using NH3 to replace fossil fuels. In this case, modifications to the equipment are necessary to limit the generation of NOx during combustion as well as to remove it from the combustion products through catalytic treatment.
The world's leading turbine suppliers such as Siemens or GE have also made moves in using hydrogen turbines with a roadmap towards running 100% hydrogen turbines such as GE's 9F.05 turbine that has been used. successfully combined operating a mixture of natural gas and hydrogen feedstocks at EnergyAustralia. Currently, there are a number of projects using hydrogen in electricity production under construction, including Ballard Power Systems and its partner Hydrogen de France, which is building the CEOG multi-Megawatt hydrogen power plant in Guiana, France [35]. Or Electricity supplier eRex built the first commercial hydrogen power plant located in Yamanashi, Japan with a capacity of 360 kW expected to come into operation in March 2022 [36].
4 Challenges for green hydrogen
Climate change is currently a global problem, a challenge for governments and industries. In order to meet commitments to combat climate change, regions/governments have promoted reductions in greenhouse gas (CO2) emissions across industries. In efforts to combat climate change, green hydrogen has become an important energy source in the world and is a central factor along with carbon capture in heat-sustaining scenarios. Earth's temperature will increase no more than 2oC by 2050.
Despite playing an important role in a decarbonized economy, the production, use and commercialization of green hydrogen faces major challenges in terms of cost and infrastructure; on technology safety and the challenge of social acceptance of green hydrogen technology.
4.1 Cost and infrastructure challenges
The cost of green hydrogen production can be quantified by the LCOH-averaged cost, which includes components such as electricity costs, investment costs and fixed operating costs. Among them, electricity costs account for the highest proportion, accounting for about 50 一 55% [38]. Renewable energy electricity prices will vary depending on geographical area and depend on the renewable energy potential of that area. Thus, countries with renewable energy potential will have a cost advantage in green hydrogen production, such as Australia, China, Chile, Germany, Morocco and the UK. Figure 110 below shows the estimated LCOH green hydrogen price in terms of technology and renewable energy power systems in 2020 in the US, Europe and Australia for a capacity scale of 10 MW for proton membrane electrolysis method. , alkaline electrolysis uses wind and solar power.
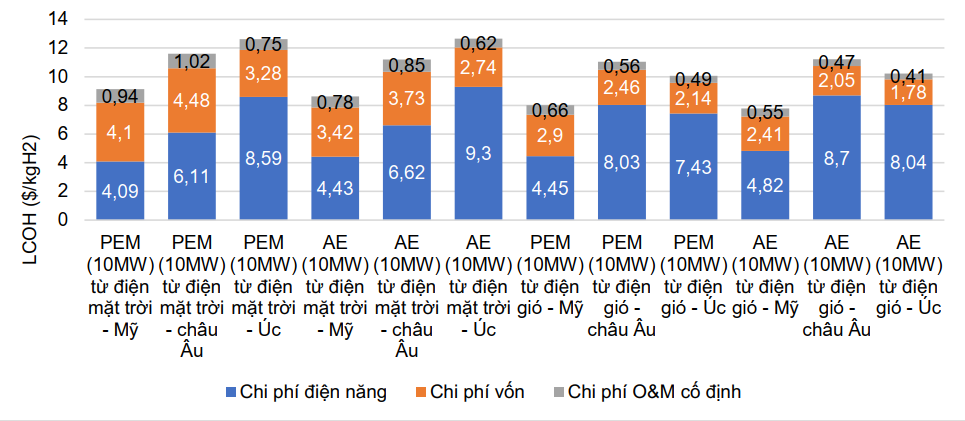
Figure 110. LCOH green hydrogen prices in the US, Europe and Australia in terms of technology and power system
renewable in 2020 [37]
It can be seen that LCOH is lowest in the US, fluctuating between 7.78 - 9.13 $/kg, while in Europe and Australia it is 11.05 一 11.61 $/kg and 10.06 respectively.一 12.66 $/kg. Traditional hydrogen production methods from fossil fuels (called gray hydrogen) cost LCOH hydrogen at about 1-2 $/kg [39] and it is predicted that this price will not change at least until 2030. For traditional production methods, the price of hydrogen will depend on the price of raw materials by region and time, for example, for the SMR method, the price of hydrogen will fluctuate depending on the price of natural gas, estimated consumes about 5 tons of natural gas for 1 ton of hydrogen product. Thus, the price of green hydrogen is at least 4 times higher than gray hydrogen. As mentioned above, electricity costs account for the majority of green hydrogen production costs, so the cost of green hydrogen production can be significantly reduced if renewable electrical energy sources are truly available in price and quantity. . One study used the Monte Carlo method to predict green hydrogen prices and suggested that LCOH green hydrogen prices would decrease over the next ten years. In 2030, the price of hydrogen produced using the SMR method is the lowest, followed by SMR combined with carbon capture and storage and coal gasification methods. The price of hydrogen produced by these methods is less than 3 $/kg. Meanwhile, the price of hydrogen produced by electrolysis fluctuates between 4-8 $/kg depending on different regions.
At present, the scale of electrolysis of green hydrogen production is still very small. The largest capacity is currently 10 MW of the electrolysis plant located in Fukoshima, equivalent to an output of 900 tons of hydrogen per year. Meanwhile, hydrogen produced by traditional methods such as natural gas steam reforming has been established for a long time, with mature technology and large capacity scale, for example the hydrogen production workshop of the Refinery. Nghi Son Oil 一 Vietnam has a capacity of about 145 thousand tons per year. For hydrogen to become a central element in a decarbonized economy, the scale of green hydrogen production must increase dramatically over the next 30 years. Besides increasing renewable energy development, increasing electrolysis scale-up and improving electrolysis technology also has a significant impact on hydrogen costs. In terms of electrolysis plant size, increasing the plant size from 1 MW to 20 MW can reduce costs by more than one-third [40]. Technologically, increasing the number of stacks along with automated processes in gigawatt-scale production facilities can reduce the cost of hydrogen production [40]. Before 2030, there will be a number of large-scale projects such as the electrolysis plant at Saudi Aramco with a capacity of 4 GW (equivalent to 238 thousand tons/year) [41] or the AREH project planned to develop. in Western Australia with a capacity of 23 GW (equivalent to 1,752 thousand tons/year) [42]. The main driver for the growth of green hydrogen is to reduce costs and increase electrolysis efficiency. In addition to supportive policies from governments, in order for the LCOH green hydrogen price to decrease to 2 $/kg, the price of renewable energy electricity must reach 0.03 $/kWh.
Besides cost issues, scaling up the hydrogen market also faces infrastructure barriers, especially infrastructure related to hydrogen transportation, storage and distribution. Being able to meet the growing demand for green hydrogen requires investment and construction of electricity transmission, distribution and storage networks as well as electrolysis systems, hydrogen pipeline systems and refueling systems. Hydrogen material. According to the International Energy Agency, by 2030, green hydrogen production will reach 88 million tons per year, which could cost $2.4 trillion and 1,238 GW of renewable energy.
Another factor that also contributes to the development of green hydrogen is carbon taxation. Bloomberg NEF commented that carbon tax should be applied to a number of industries such as: steel industry - 50 USD/ton CO2, cement industry - 78 USD/ton CO2, chemicals - 78 USD/ton CO2 [43]. A carbon tax would help green hydrogen compete with industrial fuel inputs.
4.2 Hydrogen technology safety challenges
Table 1 below presents the basic safety parameters of hydrogen compared to other fuels such as Methanol, Methane, Propane and gasoline.
Table 1. Basic safety parameters of hydrogen compared to fuels [44]
| Safety parameters | Unit | HydroH2 | Methane lCH3OH | Metha nCH4 | Propa nC3H8 | C7H16 gasoline |
| Lower explosion limit *) | Vol. % | 4.0 | 6.0 | 4.4 | 1.7 | 1.1 |
| Upper explosion limit *) | Vol. % | 77.0 | 50 (at 100°C) | 17.0 | 10.8 | 6.7 |
| Composition of flammable substances in the final mixture *) | Mol.- % | 29.5 | (12.2) | 9.5 | 4.0 | (1.9) |
| Minimum ignition energy *) | MJ | 0.017 | 0.14 | 0.29 | 0.24 | 0.24 |
| Auto-ignition temperature *) according to DIN 51794 standard | °C | 560 | 440 | 595 | 470 | 220 |
| Maximum burning velocity (laminar flow) | cm/s | 360 (at 40 Vol. %) | 43 | 37 | 47 | 30 |
| Flame temperature | °C | 2050 | 1870 | 1950 | 1925 | 2030 |
| Specific heat value | MJ/kg | 120 | 19.95 | 50 | 46.2 | 42 - 44 |
Values are taken from the CHEMSAFE, DECHEMA eV data warehouse
As seen in Table 18, compared to other fuels, hydrogen is flammable over a very wide concentration range (limit 4% to 77 vol%). When burned in air, hydrogen emits a flame that is difficult to see in daylight, because the flame has low thermal radiation and a high ultraviolet component. When a 2:1 hydrogen/oxygen mixture is formed and when the temperature reaches about 600°C, a chain reaction can begin leading to explosive propagation of the gas mixture (called explosive gas) due to The volume of water vapor produced is much higher than the initial hydrogen/oxygen mixture. Similarly, hydrogen can lead to explosive exothermic reactions in gas mixtures containing hydrogen and chlorine or fluoride gas with the product hydrogen chloride or hydrogen fluoride.
Hydrogen treatment is generally safe, but requires care and especially compliance with safety regulations, at least the following points:
- Safety standards for the production, storage, distribution and use of hydrogen: because hydrogen is like other gases such as natural gas, liquefied petroleum gas... so all standards, codes for gas, are also applied (ISO, IEC, ATEX etc.), in addition to specific national and hydrogen specific standards, for example the following standards [45]:
| ISO/TC 197 Hydrogen Technologies (20 countries recognized) | Overview of systems and equipment for hydrogen production, storage, transportation and measurement systems | IEC/TC 105 | Overview of hydrogen use for Fuel Cell (transportation sector) |
| ISO 15916 (2015) | International guidelines for the safe handling and storage of gaseous and liquid hydrogen | IEC 62282-2- 100 to IEC 62282-9- 102 ED1) | Fuel Cell standards cover many areas of safety and the environment |
| ISO 11114-4 | Hydrogen transport cylindrical tank | ASME Article KD-10 | Hydrogen pressure tank |
| ISO 19880 (2019) | Safety and performance requirements for automotive compressed hydrogen stations | SAE J2579 | Hydrogen gas combustion system |
| ISO/WD TR 15916 | Basic requirements for hydrogen system safety | CSA CHMC1 | Hydrogen gas testing method |
| CSA HPIT1 | Hydrogen system for trucks |
The regulations and standards in our country for gas substances are therefore also used, in addition, it is necessary to begin establishing standards for hydrogen appropriate to Vietnam's specific characteristics (hot climate, corrosive sea air). ...)
- To avoid explosive gas reactions, when working with hydrogen, the oxygen/hydrogen gas mixture should be sampled regularly, or oxygen should only be added to the hydrogen at the time of ignition;
- Because it is about 14 times lighter than air, hydrogen gas evaporates quickly in open spaces. Another property of hydrogen is its extremely high diffusivity. Because it is a very light gas and a very small molecule, hydrogen can diffuse into another medium, passing through many porous materials or even metals. This can cause the material to become brittle. The high diffusivity of hydrogen therefore requires the use of special materials for the containers - for example steel or austenitic coatings with diffusion barrier coatings. Modern composite materials can protect against hydrogen diffusion with appropriate facing materials [46];
- Liquefaction now plays an important part in the storage for transport of hydrogen. The most commonly used pressure tanks must have a high margin of safety and be fitted with a pressure relief valve. Ignition sources must be avoided. Because it is lighter than air, hydrogen is stored outdoors. If storage must be in a closed space, a good ventilation system with necessary hydrogen leak warning devices should be installed [46];
- When hydrogen is mixed into natural gas as a supporting gas, the safety characteristics (explosive limit, limited oxygen concentration, maximum explosion pressure, pressure increase index over time and minimum number of slots to prevent backfire) of the gas mixture is not significantly affected by the addition of hydrogen to a concentration of 10 vol% [47];
- Finally, to meet safety requirements and be licensed to build and operate a hydrogen production, storage, distribution or transportation plant, steps must be taken to analyze and identify safety measures. through current application methods such as HAZOP, LOPA.. Currently, there are specialized safety companies that provide safety surveys through the above methods, and using software equations (for example: HyRAM [45]);
- Along with hydrogen technology, the hydrogen safety industry is developing rapidly, every year there are symposiums around the world on hydrogen technology safety (for example: International Conference on Hydrogen Safety (Hysafe), Center for Hydrogen Safety Conference in America, Europe (AIChE), recently held the International Conference on Hydrogen Safety and Security ICHSS 2020 in Tokyo...) to continuously update the latest achievements in hydrogen technology safety. Experience from hydrogen industry incidents also needs to be studied to learn from the US data warehouse (US Center for hydrogen [48]; EPSC - European Process Safety Center [49]; International Association for Hydrogen Safety, HySafe...).
4.3 Challenges of social acceptance of hydrogen technology
Green hydrogen is a future type of clean energy that has a profound impact on society when widely used, for example through means of transportation (buses, trucks, cars, motorbikes...). Extensive discussions in Germany about renewable energy from wind turbines show that widespread and solid social acceptance of green hydrogen is necessary to bring a new form of energy into people's lives [96]. The same goes for hydrogen.
The latest data from the 2021 German hydrogen understanding survey [51], especially in the transport sector [52], shows that the vast majority of Germans have heard of green hydrogen, but only 1/4 know more or less details about its use in cars through fuel cells (FCBV), but are not clear about hydrogen being used in many other industrial fields such as chemistry, steelmaking, cement, and manufacturing. glass... However, most Germans are willing to support green hydrogen as a new form of clean energy knowing that it helps reduce carbon dioxide emissions to reduce atmospheric temperatures, protect clean air as well as protect the environment. such as health in densely populated cities. The survey also shows that Germans support government investment in hydrogen technology, seeing the benefits of this technology that can create new jobs, boost economic growth, and be as safe to use as fossil fuels. other. A survey in Japan on opinions on using hydrogen to replace gasoline in cars showed that the rate of assessment of the dangers of hydrogen and gasoline in cars was equal (40%) [45].
We Vietnamese people have long experience using gas in daily activities such as cooking in the oven. If hydrogen is mixed into gas as a supporting energy, there is a high possibility that people will be willing to accept its use. To achieve widespread social acceptance in the future, it is necessary to widely disseminate information about green hydrogen as a clean energy through a long-term, methodical, systematic plan that must be initiated. start as soon as possible. This plan combines many synergistic impacts on many aspects [50]:
- Political system: Disseminate policies on targets and prices to support the development of green hydrogen produced from renewable energy (wind, solar, biomass...) to gradually replace fossil fuels (for example, at low prices). FIT);,
- Community: Organize widespread information about the benefits of hydrogen in reducing CO2 emissions, protecting the environment, reducing temperatures to protect the atmosphere on media networks, through talks, and organizations. introducing and visiting green hydrogen pilot plants, soliciting opinions online, etc. The goal is to create long-term community trust in hydrogen;
- Science and technology: Update and develop hydrogen technology at research institutes and universities and introduce domestic and world achievements;
- Economy: Production and construction of hydrogen distribution systems, use for domestic industry, advantages and risks in investing in green hydrogen production, price and competitiveness with other forms of fuel such as gas combustion, gasoline, electricity from coal... on the market.
Vietnamese people do not know about green hydrogen, so far they almost only use fossil fuels such as oil, gas, and coal. There are a number of articles about green hydrogen but they only appear in specialized magazines or the science and technology pages of some newspapers. Therefore, an immediate start is to widely disseminate information on media networks about green hydrogen produced from renewable energy in neighboring Asian countries (ASEAN, China, Korea, Japan, Australia) and around the world. gender (point 2. above). In the educational program, it is necessary to supplement the subject depth with methods of preparing hydrogen through electrolysis (High School) or hydrogen technology (University/College), gradually moving towards the establishment of specialized departments. The department teaches hydrogen technology as leading countries are doing (point 3 above). Once hydrogen development policies are established and promulgated, it is necessary to organize information and discussions to increase public awareness of this new form of energy.
The above long-term impacts will help form a sense of community in our country, due to the long-term use of traditional fossil fuels, from a conservative, hesitant, and waiting attitude to a supportive, positive attitude. Participate in using hydrogen in everyday use (cars, buses, gas...) thereby helping to promote the rapid development of the hydrogen market, following the general trend of countries leading in green hydrogen technology such as Europe , North America and Asia (China, Korea, Japan, Australia).
5 Some suggestions for Hydrogen technology in Vietnam
The trend of applying and developing H2 technology worldwide is happening very strongly. Leading countries in H2 such as Germany, the US, France, Australia, Korea, Japan and China and even ASEAN countries have many open policies to develop H2 technology, moving towards mastering a part of the chain. global H2 value. In the context that Vietnam is a country with abundant renewable raw materials such as wind, sunlight, and biomass resources in the tropics, but is currently very short of electricity and fuel, the development of hydrogen technology is extremely important. necessary. Based on the advances and technological challenges as well as social acceptance barriers that the article has summarized, the authors boldly contribute a number of suggestions and recommendations to promote Hydrogen technology and the Hydrogen system. An environmentally friendly energy system for the long-term benefit of the people is as follows:
- In the near future energy strategy, Vietnam needs to research and apply advanced technology to produce hydrogen and synthesis gas (H2 + CO) such as "coal+steam gasification", biomass "gasification". , to produce heat and electricity with the purpose of replacing "pure combustion" by conventional thermal power plants with low efficiency and producing CO2 emissions that negatively affect the environment.
- Learn about the emerging low-energy hydrogen production method of methane (natural gas) pyrolysis to apply in Vietnam.
- In terms of application, it is necessary to apply advanced technologies that use hydrogen in industry, for example, using hydrogen to directly reduce iron ore in steel production technology, gradually replacing the current coal-based technology, which is both low-efficient and polluted. environmental contamination.
- In green hydrogen production technology, there needs to be a roadmap including the following steps:
- Establishment of a "Green Hydrogen Complex" (Hydro Allianz) including major domestic and specialized companies that will use green hydrogen in the future (Renewable Energy), steel, cement, cars, universities engineering, oil and gas...) to create national comprehensive resources.
- Cooperate with foreign specialized firms to build one or two pilot plants to produce green hydrogen using electricity from renewable energy (wind and sunlight resources are available in large quantities in Vietnam). Pilot plants with small capacities of about 4-10 MW use PEM and Alkaline water electrolysis technology with the purpose of gaining operating experience and training skilled personnel for the future.
- Hydrogen technology research and teaching: Establish/support the establishment of hydrogen technology teaching and research departments at universities (Petroleum University, Polytechnic University) to research/adapt hydrogen technology in conditions in Vietnam, exchanging knowledge and graduate students with world research institutes.
- Disseminate information online about green hydrogen widely in the community to create understanding and support for the use of the new form of energy.
- Vietnam especially needs to take advantage of the resources of Vietnamese and Vietnamese people abroad to connect, evaluate technology trends and move towards cooperation in technology transfer so that Vietnam has the opportunity to master green hydrogen technology in the future. near future.





