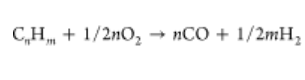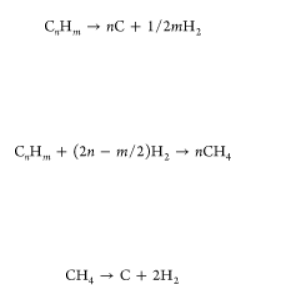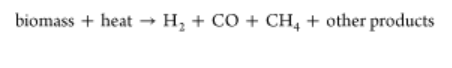Abstract
The global economic growth, the increase in the population, and advances in technology lead to an increment in the global primary energy demand. Considering that most of this energy is currently supplied by fossil fuels, a considerable amount of greenhouse gases are emitted, contributing to climate change, which is the reason why the next European Union binding agreement is focused on reducing carbon emissions using hydrogen. This study reviews different technologies for hydrogen production using renewable and non-renewable resources. Furthermore, a comparative analysis is performed on renewable-based technologies to evaluate which technologies are economically and energetically more promising. The results show how biomass-based technologies allow for a similar hydrogen yield compared to those obtained with water-based technologies but with higher energy efficiencies and lower operational costs. More specifically, biomass gasification and steam reforming obtained a proper balance between the studied parameters, with gasification being the technique that allows for higher hydrogen yields, while steam reforming is more energy-efficient. Nevertheless, the application of hydrogen as the energy vector of the future requires both the use of renewable feedstocks with a sustainable energy source. This combination would potentially produce green hydrogen while reducing carbon dioxide emissions, limiting global climate change, and, thus, achieving the so-called hydrogen economy.
1. Introduction
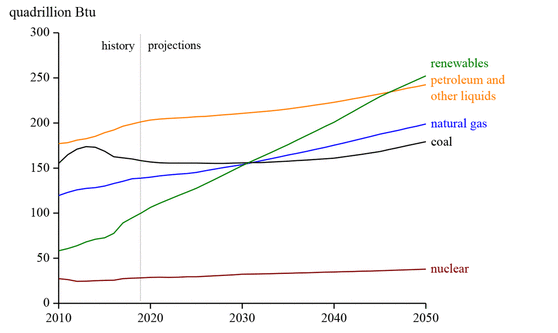
Global demand for primary energy rises by 1.3% each year to 2040, with an increasing demand for energy services (1) as a consequence of the global economic growth, the increase in the population, and advances in technology. In this sense, fossil fuels (oil, natural gas, and coal) have been widely used for energy production and are projected to remain the dominant energy source until at least 2050 (2,3) (see Figure 1). The use of fossil fuels for energy production or chemicals results in the emission of greenhouse gases, such as carbon dioxide, nitrogen oxides, and other volatile compounds, and solid particles into the atmosphere, contributing to global climate change. (4)
Figure 1
Figure 1. World primary energy consumption by energy source. This figure was adapted with permission from ref (3). Copyright 2019 U.S. Energy Information Administration (EIA).
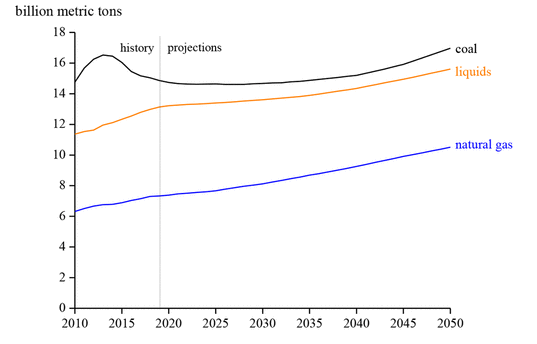
Currently, carbon-based fuels supply 85% of the entire world’s energy demand. Approximately 36 billion tons of CO2 are emitted into the atmosphere every year to meet the energy demand. Of these emissions, over 90% comes from fossil fuels, (5) and it is expected to further increase in the coming years, as shown in Figure 2.
Figure 2
Figure 2. Energy-related carbon dioxide emissions. This figure was adapted with permission from ref (3). Copyright 2019 U.S. Energy Information Administration (EIA).
Besides the strong environmental impacts, fossil fuels are ever-dwindling supplies, and oil prices wildly fluctuate, impacting profits for industries that produce and use oil and the ability of consumers to purchase goods and services. (6) Energy consumption and carbon emissions represent two crucial elements of the European Union energy strategy. (7) Different targets have been set up to ensure a reduction of both energy consumption and carbon emissions. In this sense, the next European Union binding agreement for energy efficiency target (primary energy consumption) focuses on a 30% reduction in 2030 compared to the 1990 level. (8) Nevertheless, the immediate effects of the COVID-19 pandemic on the energy system showed falls in 2020 of 5% in the global energy demand, 7% in energy-related CO2 emissions, and 18% in energy investment. Renewable energies, especially those in the power sector, have been less affected by the pandemic when compared to other fuels. (9) The World Energy Outlook 2020 has included the new net-zero emissions by 2050 scenario, which extends the sustainable development scenario based on clean energy policies and includes the first detailed International Energy Agency (IEA) modeling, setting out what additional measures would be required over the next 10 years to put global CO2 emissions on track for net zero by 2050. In this regard, a power system with net-zero emissions requires careful long-term and integrated planning. The electricity sector will play a key role in the emission reduction efforts, but low-carbon fuels, such as hydrogen, are also needed. Achieving this objective would mean an acceleration in the deployment of clean energy technologies.
On the basis of the foregoing, multiple studies have focused on developing new technologies toward renewable energy sources as an alternative to fossil fuels. (10−15) In this context, the number of countries with policies that directly support investment in hydrogen technologies is increasing. (16) Furthermore, given that the primary use of hydrogen is currently in industrial applications, it is in the interest of the European Union to shift toward the production of green hydrogen to achieve net-zero carbon emission goals. (17) Hydrogen is the most abundant gas in the universe and has the maximum energy content per unit of weight compared to any other known fuel. (18,19) Using hydrogen for energy production does not result in pollutant emissions because only heat and water vapor are produced, (20−22) reducing the emission of greenhouse gases. Besides energy applications, hydrogen is widely used in chemical and petroleum industries. (18,19,23) Despite the abundance of hydrogen, it is not available in free form in nature. (24) Nowadays, hydrogen is mainly produced through thermochemical processes using fossil fuels: hydrocarbon reforming, coal gasification, hydrocarbon pyrolysis, and plasma reforming. (18)
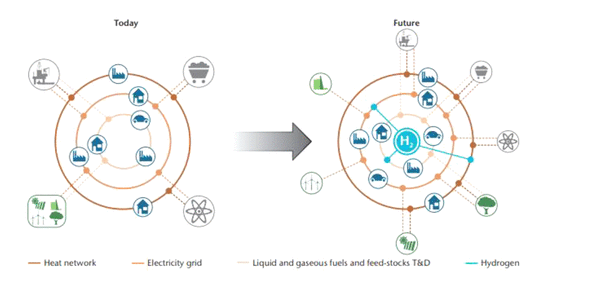
As a consequence, significant CO2 emissions are produced (around 830 million tons per year). (16) Thus, hydrogen production from renewable sources can face this problem by lowering the carbon footprint, leading to a sustainable energy system in the near future, as shown in Figure 3. In addition, to make a significant contribution toward a clean energy transition, hydrogen, apart from being produced in a cleaner way, also needs to be adopted in sectors where it is poorly represented at the moment, such as transport or buildings. (16)
Figure 3
Figure 3. Schematic representation of the energy system today and in the future. This figure was reprinted with permission from ref (25). Copyright 2015 International Energy Agency (IEA).
Hydrogen is considered the energy vector of the future. However, its sustainability depends upon the cleanness of the hydrogen production pathway and the energy used during the obtaining process. In this regard, renewable energy will play a key role during the decarbonization of the current energy system. Hence, this review presents and describes the main hydrogen production technologies, combining information from renewable and non-renewable resources. Technologies using renewable sources are also analyzed on the basis of energy efficiency, cost-effectiveness, and hydrogen yield. Furthermore, the main challenges and future perspectives for hydrogen production technologies are evaluated.
2. Technologies for Hydrogen Production
Hydrogen in molecular form can be obtained from many different resources, such as fossil fuels, biomass, and water. (16) To extract hydrogen from these sources, the energy spent must be available in an excess quantity with continuous availability. (26) Thus, tapping into the potential of renewable energies (solar, wind, sea wave, etc.) in hydrogen production technologies would allow for its sustainable production. (17,27,28) Hereafter, the main hydrogen production technologies will be set according to the raw material used: fossil fuels or renewable resources.
2.1. Hydrogen Production from Fossil Fuels
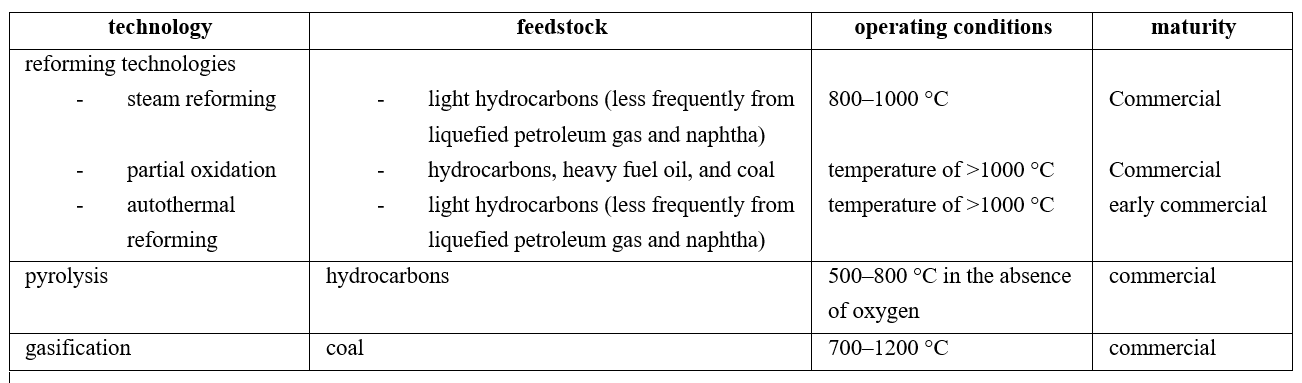
Fossil fuels remain dominant in the global hydrogen supply because production costs are strongly correlated with fuel prices, which are still maintained at acceptable levels. Currently, several mature technologies produce hydrogen from fossil fuels, with hydrocarbon reforming and pyrolysis being the most used. These techniques almost allow for the production of the actual hydrogen demand. (24) More specifically, in 2015, hydrogen was produced 48% from natural gas, 30% from petroleum, and 18% from coal. (25)Table 1 summarizes the main characteristics, including the feedstock used, operating conditions, and the maturity of each fossil-fuel-based technology described in the following subsections.
Table 1. Summary of Hydrogen Production Technologies from Fossil Fuels
2.1.1. Fossil Hydrocarbon Reforming Technologies
Hydrocarbon reforming is the most developed technique for hydrogen production. Apart from hydrocarbon, other reactants are required for this process, which could be either steam or oxygen, commonly known as the steam reforming or partial oxidation reaction, respectively. When both reactions are combined, with a net reaction enthalpy change of 0, the process is named autothermal reforming. (29)
2.1.1.1. Steam Reforming
The steam reforming reaction is the reaction of a mixture of steam and hydrocarbons at high temperatures to produce hydrogen and carbon oxides. Steam reforming extracts hydrogen from natural gas and much less frequently from liquefied petroleum gas and naphtha. (16) As stated before, the most widely used hydrocarbon reformation process is steam methane reforming from natural gas or light hydrocarbons. In this process, carbon monoxide is first produced with hydrogen, giving rise to synthesis gas (CH4 + H2O → CO + 3H2), and then through the water–gas shift reaction, carbon monoxide is converted to carbon dioxide and additional hydrogen (CO + H2O → CO2 + H2). (30) The overall methane steam reforming reaction can be represented as follows:
CH4 + 2H2O → CO2 + 4H2
The reforming reaction is highly endothermic, and a large amount of heat is required. For that reason, these reactions are typically carried out at temperatures between 800 and 1000 °C. (31) Given the high temperatures demanded to convert methane into hydrogen, they determine the need for expensive construction materials for the reformer to withstand the thermal stresses (e.g., high alloy nickel–chromium steel). (32) Coke formation and the emergence of temperature profiles in the catalyst bed would also need to be considered as other drawbacks. (33) To overcome the shortcomings of methane steam reforming, high-performance catalysts are required to maximize hydrogen produced commonly based on nickel (although noble metals are also active but too expensive for commercial application) supported on ceramic oxides or oxides stabilized by hydraulic cement. (34) Nevertheless, once these aspects are considered and minimized, the methane steam reforming produces a hydrogen-rich gas and fewer amounts of carbon dioxide, carbon monoxide, and methane. Because hydrogen is mixed with other compounds, a separation step is required for purification. In hydrogen plants, the purification is accomplished via the pressure swing adsorption system, which can produce up to 99.999% pure hydrogen with a recovery of 70–95%. (35)
2.1.1.2. Partial Oxidation
Partial oxidation is an alternative approach to steam reforming reactions. This process could operate with different feedstocks ranging from methane to heavy fuel oil and coal. (16,24) Partial oxidation is the most appropriate technology to obtain hydrogen from heavy fuel oil and coal. (29)
Partial oxidation is an exothermic process used to convert hydrocarbon fuels into a mixture of hydrogen, carbon monoxide, and other partially oxidized species. (36) One of the advantages of this process is that reactions with oxygen are highly exothermic, without any external energy source being necessary. (37) The product distribution of partial oxidation reactions depends upon the C/O ratio and is constrained by high reaction temperatures (>1000 °C); thus, partial oxidation reactions are usually carried out using heterogeneous catalysts at lower temperatures. Being cheaper than noble metals, transition-metal-based catalysts are suitable for partial oxidation reactions because of their ability to change oxidation states and adsorb reactants and intermediates onto their surface. (38) Overall, the partial oxidation reaction could be described as follows:
Thermodynamically, in this process, H2 and CO are the most abundant products above 550 °C, (39) with CO being a coke precursor, which can be removed by its oxidation toward CO2 or by the water–gas shift reaction increasing the H2 production. Partial oxidation offers several advantages, such as simple operation, lower energy consumption, and flexible feedstock. However, it still faces challenges for its industrial implementation, like the short useful life of the unit because of the high reaction exothermicity that could lead to hot spots and, consequently, catalyst deactivation by sintering. (37,40,41)
2.1.1.3. Autothermal Reforming
The autothermal reforming includes the exothermic partial oxidation with O2, which provides the energy needed for the endothermic steam reforming reactions. (42) In essence, both steam and oxygen are introduced into the reformer, leading to the reforming and oxidation reactions simultaneously to obtain a thermodynamically neutral reaction. (43) As in steam reforming or partial oxidation, catalyst selection plays a crucial role in the overall performance, with nickel-based catalysts being the most commonly used because of their effectiveness and low cost. Given the high thermal efficiency of this process, it demands lower energy than steam reforming or partial oxidation. (44) However, autothermal reforming produces a higher hydrogen yield than partial oxidation but lower than the steam reforming process.
2.1.2. Fossil Hydrocarbon Pyrolysis
Pyrolysis is a thermal decomposition process occurring in the absence of oxygen, (45) which converts different light liquid hydrocarbons into elemental carbon and hydrogen, according to eq 3.
When the thermal decomposition is from heavy residual fractions with a boiling point higher than 350 °C, the hydrogen production is reasonable to be carried out in a two-step scheme. These two steps are hydrogasification (eq 4) and subsequent cracking of methane (eq 5) because heavy residual fractions contain large quantities of sulfur and metals, which, in the case of direct decomposition, will be transferred into the coke and render it useless for further use. Although the amount of hydrogen produced is less when compared to the technologies that use light hydrocarbon fractions, this process allows for the simultaneous production of valuable byproducts, such as sulfur or metals. (46) Methane pyrolysis is widely reported in the literature because no carbon dioxide is produced because all carbon is recovered in solid form. From an energy point of view, the reaction enthalpy for hydrogen production by methane pyrolysis (methane decarbonization), 37 kJ/mol of H2, is similar to that of steam reforming (41 kJ/mol of H2) if the energy for the water vaporization is not considered. (47) In practice, for industrial processes, natural gas is used as a feedstock instead of methane; therefore, other compounds are mixed with methane, such as CO2 or H2O. These compounds also react during the pyrolysis process, thus strongly influencing product selectivity and conversion. For this process, different catalysts have been reported, ranging from metallic catalysts to carbonaceous catalysts. Ni-based catalysts have been extensively used as metal-based catalysts as a result of their activity and ability to produce filamentous carbon at moderate temperatures, while activated carbon and carbon black have been used as carbonaceous catalysts. (48)
2.1.2.1. Coal Gasification
Coal gasification is defined as the thermochemical conversion process in which coal is converted into gaseous products, including hydrogen and carbon monoxide. (49−51) This process aims to be an alternative to burning coal to reduce harmful emissions and increase the energy density of the fuel. (52) In practice, coal is converted to synthesis gas in the presence of steam and oxygen or air at high temperatures and pressures. (53−55) Apart from the main reactions, there may be other secondary reactions in which coal does not react with oxygen or steam but with other reaction products, such as carbon dioxide, through the Boudouard reaction, producing additional carbon monoxide. (49) The main problem associated with hydrogen production through coal gasification instead of other technologies that use different feedstocks is related to higher CO2 emissions given the high carbon content. For this reason, advantages are being developed coupling coal gasification with carbon-capture-based technologies. When compared to other technologies that use fossil fuels from the economic standpoint, coal gasification differs given the lower feedstock costs; however, the capital costs of the unit are higher. (56)
2.2. Hydrogen Production from Renewable Resources
Although most hydrogen is produced nowadays from hydrocarbons, renewable resources have attracted the attention to produce green hydrogen. (57) Hydrogen with a low-carbon footprint can significantly reduce energy-related CO2 emissions and contribute to limiting the global temperature rise to 2 °C. (25) In this sense, green hydrogen can be produced from water or biomass-derived compounds.
2.2.1. Hydrogen Production from Water
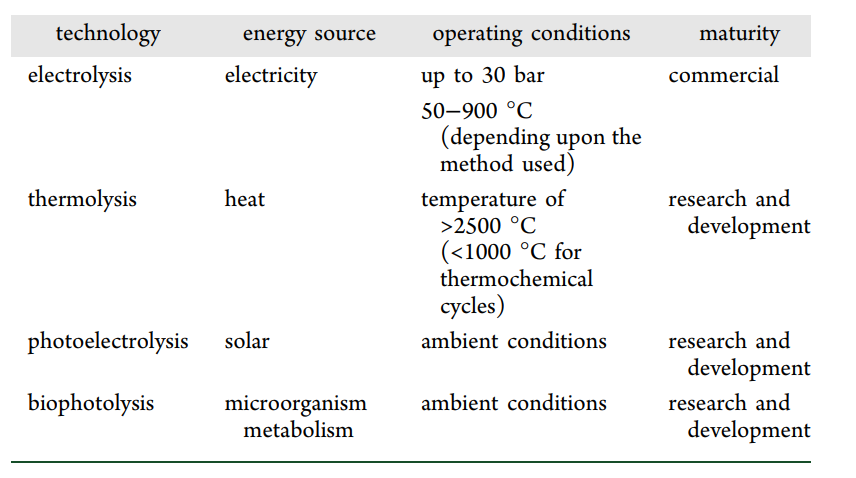
Water is the most abundant resource for hydrogen production, and it can be split into hydrogen and oxygen if enough energy is provided without harmful emissions. (58,59) Water splitting in its simplest form uses an electrical current (electrolysis) passing through two electrodes to break water into hydrogen and oxygen. (60) However, it can also be split using other energy sources, such as thermal energy (thermolysis), photonic energy (photoelectrolysis), and biophotolysis using microorganisms; (58,61) these aspects and other characteristics are summarized in Table 2.
Table 2. Summary of Hydrogen Production Technologies from Water
2.2.1.1. Electrolysis
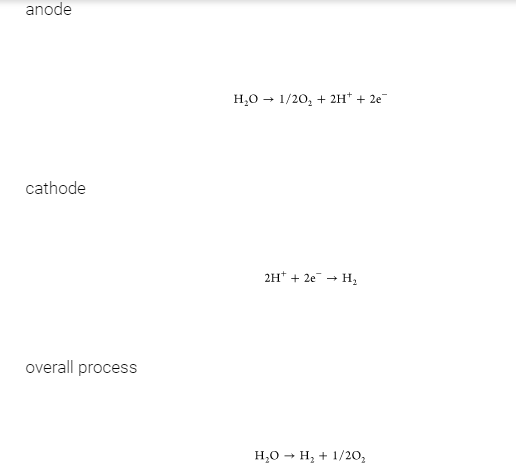
Electrolysis is one of the simplest ways to produce hydrogen from water. It can be summarized as the conversion of electric power to chemical energy in the form of hydrogen and oxygen as a byproduct with two reactions in each electrode: anode and cathode. (58,62)
Different technologies are available for water electrolysis, including alkaline water electrolysis, solid oxide electrolysis, and proton-exchange membrane electrolysis. (63,64) Alkaline water electrolysis requires a gas separator to prevent the mixture of the gas products. It uses concentrated lye as an electrolyte and non-noble metal-based electrodes (e.g., nickel). Proton-exchange membrane electrolysis uses humidified polymer membranes as the electrolyte and noble metals as electrocatalysts, such as platinum or iridium oxide. The operating temperature ranges from 50 to 80 °C, and the operating pressures can be set up to 30 bar for both technologies. Conversely, solid oxide electrolysis converts water into hydrogen and oxygen at high temperatures (700–900 °C), increasing the thermal demand. Thus, alkaline water electrolysis and proton-exchange membrane electrolysis are more promising technologies for implementation at a large scale given the lower investment cost and the higher lifetime of the unit. (63) Water electrolysis powered by renewable energy sources (e.g., wind, sea wave, and biomass (27)) is expected to enable the scale-up of hydrogen production (high purity of 99.9%) with zero CO2 emissions, allowing for the production of hydrogen onsite without transportation. (64) However, the cost of H2 produced by electrolysis is still significantly higher than that produced by fossil fuels. (65)
2.2.1.2. Thermolysis
Thermolysis is a thermochemical water-splitting process based on water decomposition to hydrogen and oxygen by heating at high temperatures. Even though this process implies an easy procedure, the water decomposition requires temperatures above 2500 °C. (60,66) The thermolysis process is reversible, and thus, one of the main challenges in the application is the separation of produced hydrogen and oxygen because the recombination of gaseous products may cause an explosive mixture. (67) The other challenge is the availability of materials, which can withstand the desired temperatures.
Against thermolysis, thermochemical water-splitting cycles proceed at lower maximum operating temperatures (usually below 1000 °C) and produce both H2 and O2 in separate steps, thereby avoiding their recombination and bypassing the need for high-temperature and costly downstream gas separation. (66) Increasing the number of cycles decreases the temperature required to split water but complicates the experimental procedure. Thermochemical cycles for water splitting can be divided into cycles with light operating conditions and high temperatures. (68) Although the sulfur iodine thermochemical cycle requires a high temperature, it is considered the most promising cycle, which is the reason why it is the most studied in the literature. On the contrary, the low-temperature cycles that are more reported are copper chlorine (Cu–Cl) and magnesium chloride (Mg–Cl) cycles. (68,69) Recent advances in these technologies are focused on using renewable energy sources, such as solar energy, or non-fossil fuel energy sources, such as nuclear energy. (58,70)
2.2.1.3. Photoelectrolysis
The process of water splitting by photoelectrolysis is like electrolysis but integrating this process with solar energy absorption in a single unit, thus contributing to the sustainability of the energy supply. Besides solar energy, this process should also be supported with electricity; thus, photonic and electrical energies are converted to chemical energy as hydrogen. (55) Water photoelectrolysis can be achieved by absorbing photons with energies greater than band gaps of semiconducting photoelectrodes, producing holes and electrons in photoelectrochemical cells. (71) The semiconductor (e.g., TiO2) uses the photons with energy greater than the semiconductor bandgap to generate electron–hole pairs that are split by the electric field, which traverses the electrolyte. (19) The performance of this system lies in the type of photon-absorbing material, surface properties, crystalline structure, corrosion resistance, and reactivity. (55)
2.2.1.4. Biophotolysis
Biophotolysis is a photonic-driven biochemical process for hydrogen production from water. (19) In direct biophotolysis, a water molecule is split into oxygen and hydrogen ions via photosynthesis using the catalytic activity of the hydrogenase enzyme under anaerobic conditions (72) of microorganisms, such as green microalgae or cyanobacteria. (18,61) One advantage of this process is that hydrogen can be produced from water in an aqueous environment at ambient conditions. It could be considered an environmentally sustainable and economically feasible method from both water and CO2 utilization perspectives. (18,24) Currently, given the low hydrogen yield, this technology requires a significant surface area to collect enough sunlight. (60,73) On the contrary, in indirect biophotolysis, carbohydrates are accumulated during the carbon dioxide fixation stage, producing oxygen. Hydrogen is produced in the next stage, where the produced substrates in the first stages are used as the carbon source, (72) thus decreasing the necessity of adding nutrients to the medium.
2.2.2. Hydrogen Production from Biomass
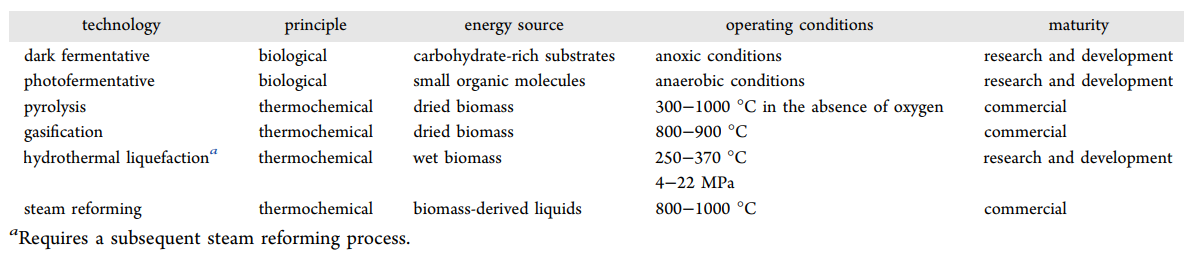
Biomass is a renewable resource of primary energy derived from plants and animal materials, (24) such as forest residues, crops, municipal solid waste, microalgae, or animal byproducts, (74,75) which are considered potential resources of fuels and chemical feedstocks. Hydrogen production is technically and economically feasible from biomass and residual wastes, given the existing technology and economic conditions in many developed countries. (76) It has been stated that biomass will cover the energy demand by more than 25% by 2050. (57) On the contrary to fossil fuels, biomass-to-energy processes reduce the CO2 emission and absorb CO2 from the natural environment, (77) leading to a neutral carbon emission scenario. There are two main processes to convert biomass into hydrogen, namely, biological and thermochemical. The thermochemical process is usually much faster than the biological process and offers a higher hydrogen yield. (18)Table 3 summarizes the main technologies for both processes, which will be subsequently developed, along with the kind of biomass used, the operational conditions, and their technological maturity.
2.2.2.1. Biological Processes
As a result of increased attention to sustainable development and waste minimization, hydrogen obtained from biological process research has increased substantially in recent years. The main biological processes used for hydrogen production include dark fermentative hydrogen production and photofermentative processes. (60,78)
For dark fermentative processes, anaerobic bacteria are used on carbohydrate-rich substrates, deprived of light and under anoxic conditions, yielding the original biomass in H2, organic acids, and CO2. (15,18,79) Because light is not required, it can produce hydrogen at any time. Biohydrogen production through the dark fermentation process occurs through biochemical reactions using enzymes at ambient temperature and pressure. (78,79) Despite the hydrogen yield being closely related to the type of substrate, inoculum, and operation conditions (e.g., temperature, pH, etc.), the process pathway metabolically restricts the maximum hydrogen yield. For example, the stoichiometric feasibility yields 12 mol of hydrogen per glucose molecule, but as a result of the thermodynamic perspective of glucose metabolism, only 4 mol of hydrogen is produced in the acetate dark fermentation. Consequently, different advances have been reported in the literature, such as the immobilization technique (78) or the use of metal ion and oxide nanoparticles to maximize the hydrogen production. (80)
On the contrary, in photofermentation, under anaerobic conditions, photosynthetic bacteria use sunlight as a source of energy and assimilate small organic molecules present in the biomass, obtaining H2 and CO2 as the byproducts, allowing for hydrogen production from a wide range of substrates. (80,81) To ensure efficient hydrogen production by photofermentation, strict control of environmental conditions is mandatory. (82) The theoretical hydrogen yield for the photofermentation process is high, along with also the high removal efficiency of the chemical oxygen demand, even though the economic viability of hydrogen production is restricted by the nitrogenase metabolism (enzyme that produces hydrogen during the N2 fixation) and the light intensity received. (83) Comparatively, the hydrogen yield is typically lower in dark conditions when compared to the yield under sunlight. (18)
2.2.2.2. Thermochemical Processes
The thermochemical processes are some of the more effective methods for producing hydrogen-rich gases from biomass. (84,85) These technologies mainly involve pyrolysis, gasification, and hydrothermal liquefaction. (24,57,74) Thermochemical conversion of dried biomass is similar to that of fossil fuels when gasification and pyrolysis are used. Both technologies produce CO and CH4, which can be processed to increase hydrogen production through the steam reforming and water–gas shift reactions. (18) In the case of wet biomass, hydrogen should be obtained by combining hydrothermal liquefaction with steam reforming.
Pyrolysis is considered the starting point of all thermochemical conversion technologies because it involves all chemical reactions to form solid, liquid, and gas as the main products with zero concentration of oxygen. (86) In recent years, advanced research using biomass for liquid fuels has been performed, ranging from pyrolysis, hydrothermal liquefaction, gasification, and biomass-to-liquid technologies to the upgrading processes. (75) Dried biomass pyrolysis is usually performed at temperatures between 300 and 1000 °C. (87,88) The pyrolytic products after thermal decomposition include biochar, bio-oil, and non-condensable gases, such as hydrogen, methane, carbon oxides, and other gaseous hydrocarbons. Product yields depend upon the operational conditions: when the temperature is below 450 °C, biochar formation is favored, whereas bio-oil is the main product at 450–800 °C. (74) Concerning operating conditions, pyrolysis is divided into fast pyrolysis and conventional pyrolysis. For hydrogen production, fast pyrolysis, which implies high temperatures and very short residence times, is preferred because the major product for conventional pyrolysis is charcoal. (57) Fast pyrolysis has been widely studied in recent years for liquid fuel production because it offers advantages in transport and storage and has relatively low investment costs and high energy efficiencies compared to other processes. There are different pyrolysis reactors described in the literature, like fixed-bed, fluidized-bed, microwave, or solar reactors, which have the advantage of using a renewable energy source during heating. (13)
Despite most pyrolysis-related studies being focused on the production of liquid biofuels, hydrogen can be directly produced at a high residence time and temperature following the reaction scheme summarized in eq 9. (89)
Additionally, formed methane and carbon monoxide can react with water to produce additional hydrogen through the steam reforming and water–gas shift reactions, respectively. To increase hydrogen produced, catalytic pyrolysis is proposed using metal- (e.g., Ni or alkali metals) or non-metal-based catalysts (e.g., activated carbon). (90) Both have demonstrated their ability to increase hydrogen production. On the contrary, to enhance the quality of the bio-oil produced, zeolites or basic materials are preferred, without costly pre-up-gradation techniques being necessary. (91)
Biomass gasification is the conversion of dry biomass into a combustible gas mixture at high temperatures (800–900 °C) to increase the hydrogen yield. (18) It is a variation of pyrolysis and, thus, is based on partial oxidation. (60) At the end of the gasification, biomass is mainly converted into CO and H2 along with some water, CO2, and CH4. Gasification requires oxidation agents, such as air, oxygen, or steam. (74,92) This technology makes it possible to obtain a much cleaner and versatile fuel than the original biomass. (85) Gasifiers can be divided into two, fixed-bed or fluidized-bed reactors. The main advantages of fixed-bed reactors are that they tend to produce a lower concentration of dust and particulates in the product gas when compared to the fluidized-bed reactor and because they are relatively more cost-effective.
Conversely, the main benefit of using fluidized-bed reactors lies in their superior heat and mass transfer between both phases, solid and gas, allowing for a homogeneous temperature and, thus, enhancing the carbon conversion and decreasing the tar formation. (92,93) To maximize syngas production, catalytic biomass gasification is also reported in the literature. Adding a catalyst during the gasification will promote the cracking reaction and reduce the activation energy, thus reducing the energy consumption. There are different types of catalysts used for this purpose, including noble metals, mineral, or alkali metal catalysts, standing out from all Ni-based catalysts given their prices and high activity, as the reason why they are extensively used for biomass catalytic gasification. (94)
Hydrothermal liquefaction of wet biomass is typically operated at moderate temperatures (250–370 °C) and high pressures (4–22 MPa) to break down the polymer structure of biomass. (74,92) The main product throughout this process is a liquid biocrude along with a gaseous stream, an aqueous phase, and a solid residue byproduct. (95) The aqueous phase can be recirculated to the hydrothermal unit to enhance the bio-oil yield. Furthermore, it could also be used to produce hydrogen-rich syngas from steam reforming. The mechanism of hydrothermal liquefaction can be divided into three main steps: depolymerization of the biomass followed by decomposition in monomers and recombination and repolymerization of reactive fragments. The reaction chemistry during the transformation of biomass into liquid products is cumbersome given the complex mixture of carbohydrates, lignin, proteins, and lipids contained in the original biomass. (75) The reactor can be either batch or continuous. Even though continuous reactors give higher feedstock conversion, most studies are focused on batch conditions. (96)
Furthermore, catalyst selection plays a key role in maximizing the bio-oil yield and decreasing the temperature and pressure required for this process. There are alkaline, acid, metal-based, and mineral catalysts whose selection depends upon the substrate used. For example, for lignocellulosic and microalgae substrates, alkaline catalysts are preferred, while for alcohols, zeolites or acid catalysts are the best option. (97) In comparison to pyrolysis, the main advantages of hydrothermal liquefaction are higher energy efficiency, lower operating temperature, and lower tar yield. (75)
The bio-oil produced from hydrothermal liquefaction and pyrolysis can be processed to increase hydrogen production. It is a liquid product that has a larger energy density than the original biomass. It is mainly composed of different kinds of oxygenated compounds, such as acids, ketones, aldehydes, and phenols, among others. (74,75,98) This complex composition depends upon the composition of the original biomass, (99,100) and it is responsible for many adverse properties of bio-oil, low heating value, corrosiveness, or immiscibility with other fuels, that make it considered a low-quality fuel. (100−102) The bio-oil can be segregated into two different fractions: one organic and the other aqueous. (103) Whereas the organic fraction is composed of nonpolar compounds and, thus, can be upgraded by hydrotreating to obtain fuels, the aqueous phase has a low value and contains mainly water but also several oxygenated organic compounds with concentrations between 15 and 60 wt %. (104) Therefore, it can be revalued by producing additional hydrogen through a catalytic reforming process. (10) However, the hydrogen yield obtained following this process is affected by the thermodynamic limitations of the water–gas shift reaction and other secondary reactions, such as methanation and coking, reducing hydrogen production. (105) Thus, the design of suitable catalysts acquires considerable importance to achieve high activity, high selectivity toward hydrogen, and low deactivation (mainly as a result of coke deposition). Moreover, the catalyst undertakes the responsibility for cracking not only C–C and C–H bonds but also O–H bonds. In this sense, noble-metal-based catalysts have been described as active and stable catalysts, but their prices are extremely high, while transition metals, such as Ni- and Co-based catalysts, have lower cost and have demonstrated suitable activity in steam reforming, which are reasons why researchers are paying much more attention to them in recent years. (20,105−107)
3. Comparative Analysis: Water versus Biomass
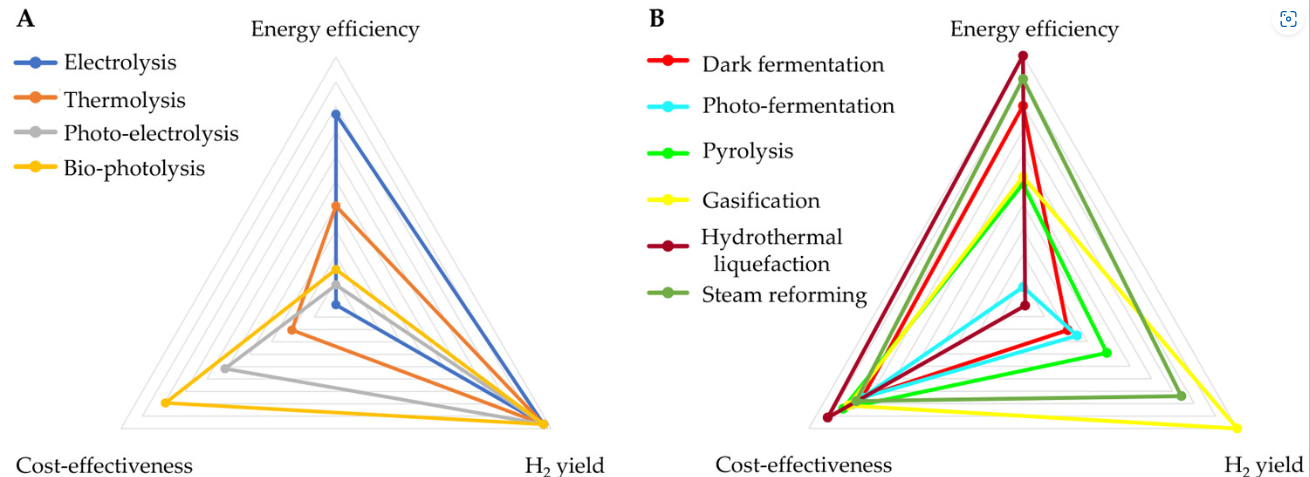
Table 4 summarizes the main advantages, drawbacks, energy efficiencies, hydrogen yields, and operational costs of the hydrogen technologies based on renewable resources. Broadly, hydrogen production from water technologies has the potential to achieve high hydrogen yields, while energy efficiency is very low to be economically competitive with other technologies. Specifically, thermolysis, photoelectrolysis, and biophotolysis have very low energy efficiencies and low cost effectiveness. The energy efficiency is relatively high for water electrolysis, but the cost effectiveness is the lowest for water-based hydrogen production technologies. Consequently, to reduce the energy requirements, researchers are focusing their attention on combining these technologies with renewable energies, reducing energy requirements, and developing suitable catalysts to make them economically competitive.
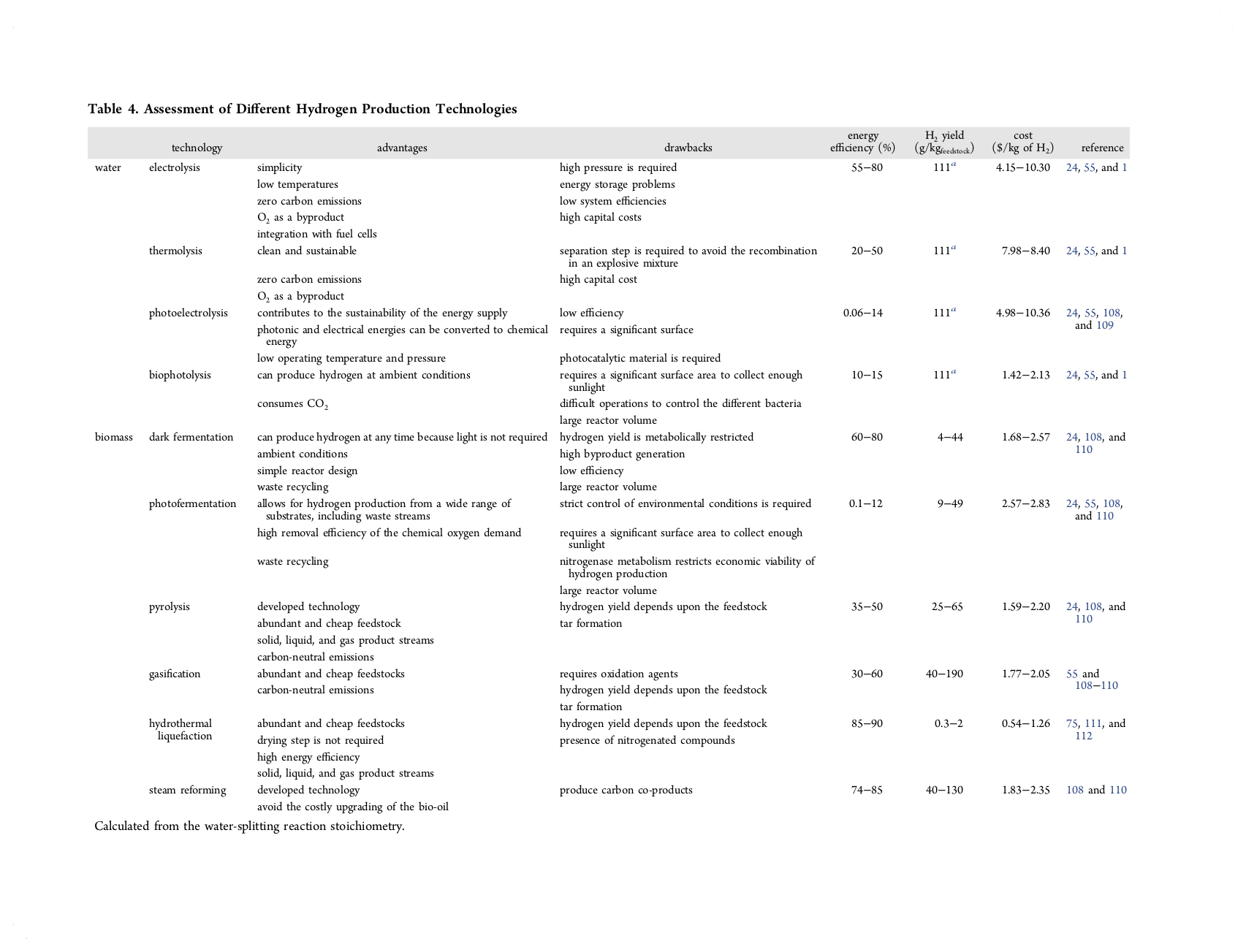
On the contrary, when biomass is used for hydrogen production, more specifically in thermochemical processes, the energy efficiency increases along with more competitive operational costs. The most energy-effective method is hydrothermal liquefaction of biomass, which also presents the highest energy efficiency, despite the hydrogen yield being limited because the main product is liquid, reaching concentrations of hydrogen in the gas streams of around 2–5%. Concerning the other thermochemical technologies, gasification and steam reforming obtained the appropriate balance between the studied parameters. Whereas gasification allows for higher hydrogen yields, the energy efficiency is favored for steam reforming, while the cost-effectiveness is similar for both. In biological processes, the hydrogen yield remains low, even though the energy efficiency for dark fermentation reaches acceptable energy efficiencies. This assumption could be discerned in Figure 4, where normalized values from Table 4 for hydrogen yields, energy efficiency, and cost effectiveness considering the maximum value for each parameter are represented for both water- and biomass-based hydrogen production technologies. Thus, to produce renewable hydrogen, the combination of biomass-to-liquid technologies with steam reforming will play a fundamental role in the energy system in the near future.
Figure 4
Figure 4. Normalized ranking comparison of different hydrogen production technologies from (A) water and (B) biomass.
The main problem with biomass technologies is that sometimes it is subjected to seasonal availability (e.g., crops or forestry residues), but this problem is less important when using waste. Moreover, producing hydrogen from wastes will play a key role in the circular economy framework, reducing the use of primary sources and waste generation, resulting in environmental preservation (neutral carbon scenario) and achieving socio-economic benefits. Regardless of the hydrogen production technology used, it is undeniable that its sustainability is linked to coupling sustainable energies during the production process.
4. Toward a Hydrogen Energy System
Hydrogen will play an essential role in the energetic environment in the near future. Aside from being a clean fuel, hydrogen is an average of 1.33 times more efficient than petroleum fuels. (113) It can also be easily integrated by adapting the existing transport and energy system, thus reducing global pollution. (114−116) Although hydrogen does not discharge greenhouse gases when used for energy applications, its sustainability depends upon the cleanness of the hydrogen production pathway and the energy used during the obtaining process. (114) In this way, the production of green hydrogen must be held using renewable resources (e.g., biomass and water) along with sustainable energy sources, such as biomass, nuclear, geothermal, solar, or wind, among other energies. (117) One problem associated with renewable energy sources, apart from the intermittence in the energy production, is the difficulty to store the electricity produced feasibly because its storage through batteries is not a viable option as a result of the limited capacity in current technology, even though many studies are working on it. (113) This problem could be solved by producing green hydrogen that can be stored and used anytime. Despite hydrogen being suitable for flameless combustion and being directly used in internal combustion engines, the worldwide hydrogen technology development is mainly focused on fuel cell technology.
The economic feasibility of hydrogen production and the security of the energy supply are the main challenges to achieve the so-called hydrogen economy, which are reasons why this issue has been included in political programs. (1,114,118) The expression “hydrogen economy” was first proposed in the past 20th century (119,120) and involves the idea of a system where hydrogen is the main energy carrier. The chief objective is to produce hydrogen at a large scale using energy sources readily available to substitute the current power economy based on fossil fuels. (116) Establishing the hydrogen economy is related to simultaneously address hydrogen production, storage, transportation, and distribution, supporting strategic policies. (121) In this regard, the strategy of policy-making decision processes in Europe is giving a primary role to hydrogen as a fuel to achieve climate action targets leading to a carbon-neutral scenario by 2050. (118) Furthermore, in the framework of the new hydrogen strategy for a climate-neutral Europe, hydrogen deployment could play a significant role in the recovery from the damaging socio-economic consequences of the recent COVID-19 emergency by creating sustainable growth and jobs. (122) In short, the importance of the hydrogen economy lies in the possibility of achieving a zero-carbon emission society, assisting the concerns about global warming and climate change, and providing sustainable alternatives.
5. Challenges and Perspectives
As a result of the environmental implications with the use of fossil fuels for hydrogen production, their use must decrease; however, even though numerous studies are focused on obtaining green hydrogen, its production is still far from being competitive. Therefore, on the basis of what is reported in the literature, some challenges must still be further researched: (1) Given that hydrogen is not in free form in nature and must be produced, the development of new production methods that reduce energy consumption and allow for its production at a large scale is required. Furthermore, it would be desirable to use water as a feedstock to reduce the environmental impact because no CO2 emissions are generated. (2) Hydrogen at ambient conditions is a gas, and therefore, it has a low volumetric density. It requires a volume of more than 3000 times higher than that of other liquid fuels to produce the same amount of energy. Moreover, the hydrogen volume must be reduced to be easily storable and transportable. (3) The flammability of hydrogen is higher than other fuels, thus bringing into question its safety. Moreover, it is an asphyxiant gas; therefore, it can lead to suffocation as a result of reducing the concentration of oxygen in the air. Thereby, certain precautions and security measures need to be considered to store or handle it safely and effectively. (4) Once hydrogen is available for end-use, the energy application for heat or electricity production must be approached as efficiently as possible. (5) The hydrogen production process bears a manufacturing cost not being a natural source, making its price 3 times higher than fossil fuels. It must also be taken into account that the storage can increase its cost even more, mainly if carried out under high-pressure technologies.
Notwithstanding the undeniable environmental benefit of using sustainable hydrogen for energy production, hydrogen energy strategies should be adopted to make hydrogen a competitive alternative to fossil fuels. More research should be performed to reach new technology improvements in hydrogen production, storage, and use. Energy efficiency will not be the only factor that determines the success or failure of each technology; other parameters, such as cost, stability, or the environmental factor will play a key role. Another roadblock that must be considered when referring to hydrogen energy is social acceptance. In some areas, hydrogen is considered a dangerous and explosive gas, affecting its reputation, especially in zones where hydrogen infrastructures are absent or no industrial application has yet been developed. Nevertheless, as future improvements on green hydrogen technologies are expected, they may enhance the renewable hydrogen options and soften the social position. (123)
6. Conclusion
Even though hydrogen is used for energy applications, it is a zero-emission energy at the end use; its sustainability is devoted to the obtaining process and the power source used during its production. This study reviews the different hydrogen production technologies available using fossil fuels or renewable resources, such as biomass and water. Currently, most hydrogen is produced from fossil fuels because production costs are correlated with fuel prices, which remain at acceptable levels. More specifically, it is mainly produced from the steam reforming of light hydrocarbons, resulting in the emission of greenhouse gases. The European Union energy strategy is focused on the reduction of carbon emissions, which is the reason why significant developments in hydrogen generation technologies from renewable resources, such as biomass and water, are taking place. The wide variety of renewable feedstocks from which hydrogen can be produced may allow for every region of the world able to produce much of its own energy, provided that these regions have access to the adequate means to carry out the conversion process. On the basis of the comparative analysis, biomass-based technologies enable similar hydrogen yields to those obtained with water-based technologies but with higher energy efficiency and lower operational costs. Therefore, renewable feedstock and sustainable energy sources for hydrogen production, substituting conventional fossil fuels and the current power system, will make it possible to achieve the so-called hydrogen economy but not without first facing technological, scientific, economic, and social roadblocks.
Author Information
Corresponding Author
Pedro J. Megía - Chemical and Environmental Engineering Group, School of Experimental Sciences and Technology (ESCET), Rey Juan Carlos University, 28933 Móstoles, Spain; Orcidhttps://orcid.org/0000-0001-7218-1275; Email: pedro.megia@urjc.es
Authors
Arturo J. Vizcaíno - Chemical and Environmental Engineering Group, School of Experimental Sciences and Technology (ESCET), Rey Juan Carlos University, 28933 Móstoles, Spain
José A. Calles - Chemical and Environmental Engineering Group, School of Experimental Sciences and Technology (ESCET), Rey Juan Carlos University, 28933 Móstoles, Spain
Alicia Carrero - Chemical and Environmental Engineering Group, School of Experimental Sciences and Technology (ESCET), Rey Juan Carlos University, 28933 Móstoles, Spain
Notes
The authors declare no competing financial interest.
Biographies
Pedro J. Megía
Pedro J. Megía received his Ph.D. degree in chemical engineering in 2020. Currently, he is a visiting professor at the Chemical and Environmental Technology Department of Rey Juan Carlos University. His research is focused on the production of renewable hydrogen from biomass-derived liquids using metal-supported mesoporous catalysts.
Arturo J. Vizcaíno
Arturo J. Vizcaíno obtained his Ph.D. in chemical engineering in 2007 at Rey Juan Carlos University (Spain), where he is currently an associate professor of chemical engineering. His research interests include the production of hydrogen from renewable resources in membrane reactors and the production of biofuels by Fischer–Tropsch synthesis.
José A. Calles
José A. Calles is a full professor in chemical engineering at Rey Juan Carlos University (Spain). His research interest is in heterogeneous catalysts and separation by membranes, both applied to hydrogen production by steam reforming and hydrogen separation in membrane reactors. He has published 61 papers with a h-index of 26.
Alicia Carrero
Alicia Carrero is a full professor in chemical engineering at Rey Juan Carlos University (Spain), She is working in hydrogen production through steam reforming of biomass-derived compounds since 2006. She has published 63 articles about heterogeneous catalysis with a h-index of 25.
Acknowledgments
This research was funded by the Regional Government of Comunidad de Madrid (Project S2018/EMT-4344) and the Spanish Ministry of Economy and Competitiveness (Project ENE2017-83696-R).
References
This article references 123 other publications.
1. International Energy Agency (IEA). World Energy Outlook 2019─Analysis; IEA: Paris, France, 2019.[Crossref], Google Scholar
2. Brockway, P. E.; Owen, A.; Brand-Correa, L. I.; Hardt, L. Estimation of Global Final-Stage Energy-Return-on-Investment for Fossil Fuels with Comparison to Renewable Energy Sources. Nat. Energy 2019, 4 (7), 612– 621, DOI: 10.1038/s41560-019-0425-z [Crossref], [CAS], Google Scholar
3. U.S. Energy Information Administration (EIA). International Energy Outlook 2019 (IEO2019); EIA: Washington, D.C., 2019.Google Scholar
4. Shindell, D.; Smith, C. J. Climate and Air-Quality Benefits of a Realistic Phase-out of Fossil Fuels. Nature 2019, 573 (7774), 408– 411, DOI: 10.1038/s41586-019-1554-z [Crossref], [PubMed], [CAS], Google Scholar
5. Uddin, K. Nuclear Energy, Environment and Public Safety: North-South Politics. Strateg. Plan. Energy Environ. 2019, 38 (4), 31– 41, DOI: 10.1080/10485236.2019.12054410 [Crossref], Google Scholar
6. Thorbecke, W. How Oil Prices Affect East and Southeast Asian Economies: Evidence from Financial Markets and Implications for Energy Security. Energy Policy 2019, 128, 628– 638, DOI: 10.1016/j.enpol.2019.01.044 [Crossref], Google Scholar
7.Bianco, V.; Cascetta, F.; Marino, A.; Nardini, S. Understanding Energy Consumption and Carbon Emissions in Europe: A Focus on Inequality Issues. Energy 2019, 170, 120– 130, DOI: 10.1016/j.energy.2018.12.120 [Crossref], Google Scholar
8. Pereira, G. I.; da Silva, P. P. Energy Efficiency Governance in the EU-28: Analysis of Institutional, Human, Financial, and Political Dimensions. Energy Effic. 2017, 10 (5), 1279– 1297, DOI: 10.1007/s12053-017-9520-9 [Crossref], Google Scholar
9. International Energy Agency (IEA). World Energy Outlook 2020; IEA: Paris, France, 2020.[Crossref], Google Scholar
10. Megía, P. J.; Carrero, A.; Calles, J. A.; Vizcaíno, A. J. Hydrogen Production from Steam Reforming of Acetic Acid as a Model Compound of the Aqueous Fraction of Microalgae HTL Using Co-M/SBA-15 (M: Cu, Ag, Ce, Cr) Catalysts. Catalysts 2019, 9 (12), 1013, DOI: 10.3390/catal9121013 [Crossref], [CAS], Google Scholar
11. Gutiérrez Ortiz, F. J.; Kruse, A.; Ramos, F.; Ollero, P. Integral Energy Valorization of Municipal Solid Waste Reject Fraction to Biofuels. Energy Convers. Manage. 2019, 180, 1167– 1184, DOI: 10.1016/j.enconman.2018.10.085 [Crossref], [CAS], Google Scholar
12. García Vera, Y. E.; Dufo-López, R.; Bernal-Agustín, J. L. Energy Management in Microgrids with Renewable Energy Sources: A Literature Review. Appl. Sci. 2019, 9 (18), 3854, DOI: 10.3390/app9183854 [Crossref], Google Scholar
13. Guedes, R. E.; Luna, A. S.; Torres, A. R. Operating Parameters for Bio-oil Production in Biomass Pyrolysis: A Review. J. Anal. Appl. Pyrolysis 2018, 129, 134– 149, DOI: 10.1016/j.jaap.2017.11.019 [Crossref], [CAS], Google Scholar
14.Abdalla, A. M.; Hossain, S.; Nisfindy, O. B.; Azad, A. T.; Dawood, M.; Azad, A. K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manage. 2018, 165, 602– 627, DOI: 10.1016/j.enconman.2018.03.088 [Crossref], [CAS], Google Scholar
15.Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Cleaner Prod. 2019, 218, 835– 849, DOI: 10.1016/j.jclepro.2019.02.046 [Crossref], [CAS], Google Scholar
16.nternational Energy Agency (IEA). Future of Hydrogen; IEA: Paris, France, 2019.[Crossref], Google Scholar
17. Handwerker, M.; Wellnitz, J.; Marzbani, H. Comparison of Hydrogen Powertrains with the Battery Powered Electric Vehicle and Investigation of Small-Scale Local Hydrogen Production Using Renewable Energy. Hydrogen 2021, 2 (1), 76– 100, DOI: 10.3390/hydrogen2010005 [Crossref], Google Scholar
18.Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K. R. Hydrogen as an Energy Vector. Renewable Sustainable Energy Rev. 2020, 120, 109620, DOI: 10.1016/j.rser.2019.109620 [Crossref], [CAS], Google Scholar
19. Dincer, I. Green Methods for Hydrogen Production. Int. J. Hydrogen Energy 2012, 37 (2), 1954– 1971, DOI: 10.1016/j.ijhydene.2011.03.173 [Crossref], [CAS], Google Scholar
20. Vizcaíno, A. J.; Carrero, A.; Calles, J. A. Hydrogen Production from Bioethanol. In Hydrogen Production: Prospects and Processes; Nova Science Publishers, Inc.: New York, 2012; pp 274– 294.Google Scholar
21. International Energy Agency (IEA). Hydrogen Production and Storage: R&D Priorities and Gaps; IEA: Paris, France, 2006.Google Scholar
22. Ruocco, C.; Palma, V.; Ricca, A. Kinetics of Oxidative Steam Reforming of Ethanol Over Bimetallic Catalysts Supported on CeO2–SiO2: A Comparative Study. Top. Catal. 2019, 62 (5–6), 467– 478, DOI: 10.1007/s11244-019-01173-2 [Crossref], [CAS], Google Scholar
23. Wang, Y.; Wang, C.; Chen, M.; Tang, Z.; Yang, Z.; Hu, J.; Zhang, H. Hydrogen Production from Steam Reforming Ethanol over Ni/Attapulgite Catalysts─Part I: Effect of Nickel Content. Fuel Process. Technol. 2019, 192, 227– 238, DOI: 10.1016/j.fuproc.2019.04.031 [Crossref], [CAS], Google Scholar
24. Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renewable Sustainable Energy Rev. 2017, 67, 597– 611, DOI: 10.1016/j.rser.2016.09.044 [Crossref], [CAS], Google Scholar
25. International Energy Agency (IEA). Technology Roadmap: Hydrogen and Fuel Cells; IEA: Paris, France, 2015.[Crossref], Google Scholar
26. Iqbal, W.; Yumei, H.; Abbas, Q.; Hafeez, M.; Mohsin, M.; Fatima, A.; Jamali, M. A.; Jamali, M.; Siyal, A.; Sohail, N. Assessment of Wind Energy Potential for the Production of Renewable Hydrogen in Sindh Province of Pakistan. Processes 2019, 7 (4), 196, DOI: 10.3390/pr7040196 [Crossref], [CAS], Google Scholar
27. Franzitta, V.; Curto, D.; Milone, D.; Trapanese, M. Energy Saving in Public Transport Using Renewable Energy. Sustainability 2017, 9 (1), 106, DOI: 10.3390/su9010106 [Crossref], [CAS], Google Scholar
28. Boujjat, H.; Rodat, S.; Abanades, S. Techno-Economic Assessment of Solar-Driven Steam Gasification of Biomass for Large-Scale Hydrogen Production. Processes 2021, 9 (3), 462, DOI: 10.3390/pr9030462 [Crossref], [CAS], Google Scholar
29. Chen, H. L.; Lee, H. M.; Chen, S. H.; Chao, Y.; Chang, M. B. Review of Plasma Catalysis on Hydrocarbon Reforming for Hydrogen Production-Interaction, Integration, and Prospects. Appl. Catal., B 2008, 85 (1–2), 1– 9, DOI: 10.1016/j.apcatb.2008.06.021 [Crossref], [CAS], Google Scholar
30. Lipman, T. E. Hydrogen Production Science and Technology. In Fuel Cells and Hydrogen Production: A Volume in the Encyclopedia of Sustainability Science and Technology, 2nd ed.; Lipman, T. E., Weber, A. Z., Eds.; Springer: New York, 2019; pp 783– 798, DOI: 10.1007/978-1-4939-7789-5_755 .[Crossref], Google Scholar
31. Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen Production through Sorption-Enhanced Steam Methane Reforming and Membrane Technology: A Review. Energy 2008, 33 (4), 554– 570, DOI: 10.1016/j.energy.2007.10.018 [Crossref], [CAS], Google Scholar
32. Amiri, T. Y.; Ghasemzageh, K.; Iulianelli, A. Membrane Reactors for Sustainable Hydrogen Production through Steam Reforming of Hydrocarbons: A Review. Chem. Eng. Process. 2020, 157, 108148, DOI: 10.1016/j.cep.2020.108148 [Crossref], [CAS], Google Scholar
33. Mbodji, M.; Commenge, J. M.; Falk, L.; Di Marco, D.; Rossignol, F.; Prost, L.; Valentin, S.; Joly, R.; Del-Gallo, P. Steam Methane Reforming Reaction Process Intensification by Using a Millistructured Reactor: Experimental Setup and Model Validation for Global Kinetic Reaction Rate Estimation. Chem. Eng. J. 2012, 207–208, 871– 884, DOI: 10.1016/j.cej.2012.07.117 [Crossref], [CAS], Google Scholar
34. Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10 (3), 352, DOI: 10.3390/catal10030352 [Crossref], [CAS], Google Scholar
35. International Energy Agency Greenhouse Gas R&D Programme (IEAGHG). Techno-Economic Evaluation of SMR Based Standalone (Merchant) Hydrogen Plant with CCS; IEAGHG: Cheltenham, U.K., 2017; Technical Report 2017/2.Google Scholar
36. Hariharan, D.; Yang, R.; Zhou, Y.; Gainey, B.; Mamalis, S.; Smith, R. E.; Lugo-Pimentel, M. A.; Castaldi, M. J.; Gill, R.; Davis, A.; Modroukas, D.; Lawler, B. Catalytic Partial Oxidation Reformation of Diesel, Gasoline, and Natural Gas for Use in Low Temperature Combustion Engines. Fuel 2019, 246, 295– 307, DOI: 10.1016/j.fuel.2019.02.003 [Crossref], [CAS], Google Scholar
37. Ma, R.; Xu, B.; Zhang, X. Catalytic Partial Oxidation (CPOX) of Natural Gas and Renewable Hydrocarbons/Oxygenated Hydrocarbons─A Review. Catal. Today 2019, 338, 18– 30, DOI: 10.1016/j.cattod.2019.06.025 [Crossref], [CAS], Google Scholar
38. Arku, P.; Regmi, B.; Dutta, A. A Review of Catalytic Partial Oxidation of Fossil Fuels and Biofuels: Recent Advances in Catalyst Development and Kinetic Modelling. Chem. Eng. Res. Des. 2018, 136, 385– 402, DOI: 10.1016/j.cherd.2018.05.044 [Crossref], [CAS], Google Scholar
39. Batista da Silva, R.; Brandão, S. T.; Lucotti, A.; Tommasini, M. S.; Castiglioni, C.; Groppi, G.; Beretta, A. Chemical Pathways in the Partial Oxidation and Steam Reforming of Acetic Acid over a Rh-Al2O3 Catalyst. Catal. Today 2017, 289, 162– 172, DOI: 10.1016/j.cattod.2016.08.018 [Crossref], [CAS], Google Scholar
40. Pirez, C.; Fang, W.; Capron, M.; Paul, S.; Jobic, H.; Dumeignil, F.; Jalowiecki-Duhamel, L. Steam Reforming, Partial Oxidation and Oxidative Steam Reforming for Hydrogen Production from Ethanol over Cerium Nickel Based Oxyhydride Catalyst. Appl. Catal., A 2016, 518, 78– 86, DOI: 10.1016/j.apcata.2015.10.035 [Crossref], [CAS], Google Scholar
41. Sawatmongkhon, B.; Theinnoi, K.; Wongchang, T.; Haoharn, C.; Wongkhorsub, C.; Sukjit, E. Modeling of Hydrogen Production from Catalytic Partial Oxidation of Ethanol over a Platinum–Rhodium-Supported Catalyst. Energy Fuels 2021, 35 (5), 4404– 4417, DOI: 10.1021/acs.energyfuels.0c04125 [ACS Full Text ACS Full Text], [CAS], Google Scholar
42. Luneau, M.; Gianotti, E.; Meunier, F. C.; Mirodatos, C.; Puzenat, E.; Schuurman, Y.; Guilhaume, N. Deactivation Mechanism of Ni Supported on Mg-Al Spinel during Autothermal Reforming of Model Biogas. Appl. Catal., B 2017, 203, 289– 299, DOI: 10.1016/j.apcatb.2016.10.023 [Crossref], [CAS], Google Scholar
43. Baruah, R.; Dixit, M.; Basarkar, P.; Parikh, D.; Bhargav, A. Advances in Ethanol Autothermal Reforming. Renewable Sustainable Energy Rev. 2015, 51, 1345– 1353, DOI: 10.1016/j.rser.2015.07.060 [Crossref], [CAS], Google Scholar
44. Tariq, R.; Maqbool, F.; Abbas, S. Z. Small-Scale Production of Hydrogen via Auto-Thermal Reforming in an Adiabatic Packed Bed Reactor: Parametric Study and Reactor’s Optimization through Response Surface Methodology. Comput. Chem. Eng. 2021, 145, 107192, DOI: 10.1016/j.compchemeng.2020.107192 [Crossref], [CAS], Google Scholar
45. Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33– 86, DOI: 10.1016/j.pecs.2017.05.004 [Crossref], Google Scholar
46. Muradov, N. Z. How to Produce Hydrogen from Fossil Fuels without CO2 Emission. Int. J. Hydrogen Energy 1993, 18 (3), 211– 215, DOI: 10.1016/0360-3199(93)90021-2 [Crossref], [CAS], Google Scholar
47. Schneider, S.; Bajohr, S.; Graf, F.; Kolb, T. State of the Art of Hydrogen Production via Pyrolysis of Natural Gas. ChemBioEng Rev. 2020, 7 (5), 150– 158, DOI: 10.1002/cben.202000014 [Crossref], [CAS], Google Scholar
48. Abbas, H. F.; Wan Daud, W. M. A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35 (3), 1160– 1190, DOI: 10.1016/j.ijhydene.2009.11.036 [Crossref], [CAS], Google Scholar
49. Stańczyk, K.; Kapusta, K.; Wiatowski, M.; Świądrowski, J.; Smoliński, A.; Rogut, J.; Kotyrba, A. Experimental Simulation of Hard Coal Underground Gasification for Hydrogen Production. Fuel 2012, 91 (1), 40– 50, DOI: 10.1016/j.fuel.2011.08.024 [Crossref], [CAS], Google Scholar
50. Sutardi, T.; Wang, L.; Karimi, N.; Paul, M. C. Utilization of H2O and CO2 in Coal Particle Gasification with an Impact of Temperature and Particle Size. Energy Fuels 2020, 34 (10), 12841– 12852, DOI: 10.1021/acs.energyfuels.0c02280 [ACS Full Text ACS Full Text], [CAS], Google Scholar
51. Emami Taba, L.; Irfan, M. F.; Wan Daud, W. A. M.; Chakrabarti, M. H. The Effect of Temperature on Various Parameters in Coal, Biomass and CO-Gasification: A Review. Renewable Sustainable Energy Rev. 2012, 16 (8), 5584– 5596, DOI: 10.1016/j.rser.2012.06.015 [Crossref], [CAS], Google Scholar
52. Seyitoglu, S. S.; Dincer, I.; Kilicarslan, A. Energy and Exergy Analyses of Hydrogen Production by Coal Gasification. Int. J. Hydrogen Energy 2017, 42 (4), 2592– 2600, DOI: 10.1016/j.ijhydene.2016.08.228 [Crossref], [CAS], Google Scholar
53. Stiegel, G. J.; Ramezan, M. Hydrogen from Coal Gasification: An Economical Pathway to a Sustainable Energy Future. Int. J. Coal Geol. 2006, 65 (3–4), 173– 190, DOI: 10.1016/j.coal.2005.05.002 [Crossref], [CAS], Google Scholar
54. Mularski, J.; Pawlak-Kruczek, H.; Modlinski, N. A Review of Recent Studies of the CFD Modelling of Coal Gasification in Entrained Flow Gasifiers, Covering Devolatilization, Gas-Phase Reactions, Surface Reactions, Models and Kinetics. Fuel 2020, 271, 117620, DOI: 10.1016/j.fuel.2020.117620 [Crossref], [CAS], Google Scholar
55. Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40 (34), 11094– 11111, DOI: 10.1016/j.ijhydene.2014.12.035 [Crossref], [CAS], Google Scholar
56. Acar, C.; Dincer, I. Comparative Assessment of Hydrogen Production Methods from Renewable and Non-Renewable Sources. Int. J. Hydrogen Energy 2014, 39 (1), 1– 12, DOI: 10.1016/j.ijhydene.2013.10.060 [Crossref], [CAS], Google Scholar
57. Hosseini, S. E.; Wahid, M. A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renewable Sustainable Energy Rev. 2016, 57, 850– 866, DOI: 10.1016/j.rser.2015.12.112 [Crossref], [CAS], Google Scholar
58. Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manage. 2020, 205, 112182, DOI: 10.1016/j.enconman.2019.112182 [Crossref], [CAS], Google Scholar
59.Bolt, A.; Dincer, I.; Agelin-Chaab, M. A Review of Unique Aluminum–Water Based Hydrogen Production Options. Energy Fuels 2021, 35 (2), 1024– 1040, DOI: 10.1021/acs.energyfuels.0c03674 [ACS Full Text ACS Full Text], [CAS], Google Scholar
60. Holladay, J. D.; Hu, J.; King, D. L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139 (4), 244– 260, DOI: 10.1016/j.cattod.2008.08.039 [Crossref], [CAS], Google Scholar
61. Dincer, I.; Zamfirescu, C. Sustainable Hydrogen Production Options and the Role of IAHE. Int. J. Hydrogen Energy 2012, 37 (21), 16266– 16286, DOI: 10.1016/j.ijhydene.2012.02.133 [Crossref], [CAS], Google Scholar
62. Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42 (52), 30470– 30492, DOI: 10.1016/j.ijhydene.2017.10.045 [Crossref], [CAS], Google Scholar
63. Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8 (2), 248, DOI: 10.3390/pr8020248 [Crossref], [CAS], Google Scholar
64. Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39 (3), 390– 394, DOI: 10.1016/S1872-2067(17)62949-8 [Crossref], [CAS], Google Scholar
65. Jia, J.; Seitz, L. C.; Benck, J. D.; Huo, Y.; Chen, Y.; Ng, J. W. D.; Bilir, T.; Harris, J. S.; Jaramillo, T. F. Solar Water Splitting by Photovoltaic-Electrolysis with a Solar-to-Hydrogen Efficiency over 30%. Nat. Commun. 2016, 7, 13237, DOI: 10.1038/ncomms13237 [Crossref], [PubMed], [CAS], Google Scholar
66. Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3 (3), 63, DOI: 10.3390/chemengineering3030063 [Crossref], [CAS], Google Scholar
67. Acar, C.; Bicer, Y.; Demir, M. E.; Dincer, I. Transition to a New Era with Light-Based Hydrogen Production for a Carbon-Free Society: An Overview. Int. J. Hydrogen Energy 2019, 44 (47), 25347– 25364, DOI: 10.1016/j.ijhydene.2019.08.010 [Crossref], [CAS], Google Scholar
68. Oruc, O.; Dincer, I. Assessing the Potential of Thermochemical Water Splitting Cycles: A Bridge towards for Clean and Sustainable Hydrogen Generation. Fuel 2021, 286, 119325, DOI: 10.1016/j.fuel.2020.119325 [Crossref], [CAS], Google Scholar
69. El-Emam, R. S.; Ozcan, H.; Zamfirescu, C. Updates on Promising Thermochemical Cycles for Clean Hydrogen Production Using Nuclear Energy. J. Cleaner Prod. 2020, 262, 121424, DOI: 10.1016/j.jclepro.2020.121424 [Crossref], [CAS], Google Scholar
70. Rosen, M. A. Advances in Hydrogen Production by Thermochemical Water Decomposition: A Review. Energy 2010, 35 (2), 1068– 1076, DOI: 10.1016/j.energy.2009.06.018 [Crossref], [CAS], Google Scholar
71. Chen, Z.; Jaramillo, T. F.; Deutsch, T. G.; Kleiman-Shwarsctein, A.; Forman, A. J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; McFarland, E. W.; Domen, K.; Miller, E. L.; Turner, J. A.; Dinh, H. N. Accelerating Materials Development for Photoelectrochemical Hydrogen Production: Standards for Methods, Definitions, and Reporting Protocols. J. Mater. Res. 2010, 25 (1), 3– 16, DOI: 10.1557/JMR.2010.0020 [Crossref], [CAS], Google Scholar
72. Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45 (43), 22492– 22512, DOI: 10.1016/j.ijhydene.2020.06.182 [Crossref], [CAS], Google Scholar
73. Franzitta, V.; Curto, D.; Rao, D.; Viola, A. Hydrogen Production from Sea Wave for Alternative Energy Vehicles for Public Transport in Trapani (Italy). Energies 2016, 9 (10), 850, DOI: 10.3390/en9100850 [Crossref], [CAS], Google Scholar
74. Ong, H. C.; Chen, W. H.; Farooq, A.; Gan, Y. Y.; Lee, K. T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renewable Sustainable Energy Rev. 2019, 113, 109266, DOI: 10.1016/j.rser.2019.109266 [Crossref], [CAS], Google Scholar
75. Gollakota, A. R. K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renewable Sustainable Energy Rev. 2018, 81, 1378– 1392, DOI: 10.1016/j.rser.2017.05.178 [Crossref], Google Scholar
76. Shahabuddin, M.; Krishna, B. B.; Bhaskar, T.; Perkins, G. Advances in the Thermo-Chemical Production of Hydrogen from Biomass and Residual Wastes: Summary of Recent Techno-Economic Analyses. Bioresour. Technol. 2020, 299, 122557, DOI: 10.1016/j.biortech.2019.122557 [Crossref], [PubMed], [CAS], Google Scholar
77. Uddin, M. N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M. G.; Mahlia, T. M. I.; Ashrafur, S. M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11 (11), 3115, DOI: 10.3390/en11113115 [Crossref], [CAS], Google Scholar
78. Zhang, C.; Kang, X.; Liang, N.; Abdullah, A. Improvement of Biohydrogen Production from Dark Fermentation by Cocultures and Activated Carbon Immobilization. Energy Fuels 2017, 31 (11), 12217– 12222, DOI: 10.1021/acs.energyfuels.7b02035 [ACS Full Text ACS Full Text], [CAS], Google Scholar
79. Bundhoo, Z. M. A. Potential of Bio-Hydrogen Production from Dark Fermentation of Crop Residues: A Review. Int. J. Hydrogen Energy 2019, 44 (32), 17346– 17362, DOI: 10.1016/j.ijhydene.2018.11.098 [Crossref], [CAS], Google Scholar
80. Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strateg. Rev. 2019, 24, 27– 37, DOI: 10.1016/j.esr.2019.01.001 [Crossref], Google Scholar
81. Eroglu, E.; Melis, A. Photobiological Hydrogen Production: Recent Advances and State of the Art. Bioresour. Technol. 2011, 102 (18), 8403– 8413, DOI: 10.1016/j.biortech.2011.03.026 [Crossref], [PubMed], [CAS], Google Scholar
82. Argun, H.; Kargi, F. Bio-Hydrogen Production by Different Operational Modes of Dark and Photo-Fermentation: An Overview. Int. J. Hydrogen Energy 2011, 36, 7443– 7459, DOI: 10.1016/j.ijhydene.2011.03.116 [Crossref], [CAS], Google Scholar
83. Lee, H. S.; Vermaas, W. F. J.; Rittmann, B. E. Biological Hydrogen Production: Prospects and Challenges. Trends Biotechnol. 2010, 28 (5), 262– 271, DOI: 10.1016/j.tibtech.2010.01.007 [Crossref], [PubMed], [CAS], Google Scholar
84. Hossain, M. A.; Jewaratnam, J.; Ganesan, P. Prospect of Hydrogen Production from Oil Palm Biomass by Thermochemical Process─A Review. Int. J. Hydrogen Energy 2016, 41 (38), 16637– 16655, DOI: 10.1016/j.ijhydene.2016.07.104 [Crossref], [CAS], Google Scholar
85. Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-Based Hydrogen Production: A Review and Analysis. Int. J. Hydrogen Energy 2009, 34 (21), 8799– 8817, DOI: 10.1016/j.ijhydene.2009.08.078 [Crossref], [CAS], Google Scholar
86. Patel, M.; Zhang, X.; Kumar, A. Techno-Economic and Life Cycle Assessment on Lignocellulosic Biomass Thermochemical Conversion Technologies: A Review. Renewable Sustainable Energy Rev. 2016, 53, 1486– 1499, DOI: 10.1016/j.rser.2015.09.070 [Crossref], [CAS], Google Scholar
87. Mutsengerere, S.; Chihobo, C. H.; Musademba, D.; Nhapi, I. A Review of Operating Parameters Affecting Bio-oil Yield in Microwave Pyrolysis of Lignocellulosic Biomass. Renewable Sustainable Energy Rev. 2019, 104, 328– 336, DOI: 10.1016/j.rser.2019.01.030 [Crossref], [CAS], Google Scholar
88. Zhang, H.; Gao, Z.; Ao, W.; Li, J.; Liu, G.; Fu, J.; Ran, C.; Mao, X.; Kang, Q.; Liu, Y.; Dai, J. Microwave-Assisted Pyrolysis of Textile Dyeing Sludge Using Different Additives. J. Anal. Appl. Pyrolysis 2017, 127, 140– 149, DOI: 10.1016/j.jaap.2017.08.014 [Crossref], [CAS], Google Scholar
89. Ni, M.; Leung, D. Y. C.; Leung, M. K. H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87 (5), 461– 472, DOI: 10.1016/j.fuproc.2005.11.003 [Crossref], [CAS], Google Scholar
90. Suprianto, T.; Winarto; Wijayanti, W.; Wardana, I. N. G. Synergistic Effect of Curcumin and Activated Carbon Catalyst Enhancing Hydrogen Production from Biomass Pyrolysis. Int. J. Hydrogen Energy 2021, 46 (10), 7147– 7164, DOI: 10.1016/j.ijhydene.2020.11.211 [Crossref], [CAS], Google Scholar
91. Goyal, H. B.; Seal, D.; Saxena, R. C. Bio-Fuels from Thermochemical Conversion of Renewable Resources: A Review. Renewable Sustainable Energy Rev. 2008, 12 (2), 504– 517, DOI: 10.1016/j.rser.2006.07.014 [Crossref], [CAS], Google Scholar
92. Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34 (11), 13438– 13455, DOI: 10.1021/acs.energyfuels.0c02900 [ACS Full Text ACS Full Text], [CAS], Google Scholar
93. Safarian, S.; Unnpórsson, R.; Richter, C. A Review of Biomass Gasification Modelling. Renewable Sustainable Energy Rev. 2019, 110, 378– 391, DOI: 10.1016/j.rser.2019.05.003 [Crossref], [CAS], Google Scholar
94. Ren, J.; Cao, J. P.; Zhao, X. Y.; Yang, F. L.; Wei, X. Y. Recent Advances in Syngas Production from Biomass Catalytic Gasification: A Critical Review on Reactors, Catalysts, Catalytic Mechanisms and Mathematical Models. Renewable Sustainable Energy Rev. 2019, 116, 109426, DOI: 10.1016/j.rser.2019.109426 [Crossref], [CAS], Google Scholar
95. Rowbotham, J. S.; Dyer, P. W.; Greenwell, H. C.; Theodorou, M. K. Thermochemical Processing of Macroalgae: A Late Bloomer in the Development of Third-Generation Biofuels?. Biofuels 2012, 3 (4), 441– 461, DOI: 10.4155/bfs.12.29 [Crossref], [CAS], Google Scholar
96. Elliott, D. C.; Biller, P.; Ross, A. B.; Schmidt, A. J.; Jones, S. B. Hydrothermal Liquefaction of Biomass: Developments from Batch to Continuous Process. Bioresour. Technol. 2015, 178, 147– 156, DOI: 10.1016/j.biortech.2014.09.132 [Crossref], [PubMed], [CAS], Google Scholar
97. Basar, I. A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A Review on Key Design and Operational Parameters to Optimize and Develop Hydrothermal Liquefaction of Biomass for Biorefinery Applications. Green Chem. 2021, 23 (4), 1404– 1446, DOI: 10.1039/D0GC04092D [Crossref], [CAS], Google Scholar
98. Kalogiannis, K. G.; Stefanidis, S. D.; Lappas, A. A. Catalyst Deactivation, Ash Accumulation and Bio-oil Deoxygenation during Ex Situ Catalytic Fast Pyrolysis of Biomass in a Cascade Thermal-Catalytic Reactor System. Fuel Process. Technol. 2019, 186, 99– 109, DOI: 10.1016/j.fuproc.2018.12.008 [Crossref], [CAS], Google Scholar
99. Esteban-Díez, G.; Gil, M. V.; Pevida, C.; Chen, D.; Rubiera, F. Effect of Operating Conditions on the Sorption Enhanced Steam Reforming of Blends of Acetic Acid and Acetone as Bio-oil Model Compounds. Appl. Energy 2016, 177, 579– 590, DOI: 10.1016/j.apenergy.2016.05.149 [Crossref], [CAS], Google Scholar
100. Gao, N.; Quan, C.; Ma, Z.; Wu, C. Thermal Characteristics of Biomass Pyrolysis Oil and Potential Hydrogen Production by Catalytic Steam Reforming. Energy Fuels 2018, 32 (4), 5234– 5243, DOI: 10.1021/acs.energyfuels.8b00365 [ACS Full Text ACS Full Text], [CAS], Google Scholar
101. Bridgwater, A. V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68– 94, DOI: 10.1016/j.biombioe.2011.01.048 [Crossref], [CAS], Google Scholar
102. Xiu, S.; Shahbazi, A. Bio-oil Production and Upgrading Research: A Review. Renewable Sustainable Energy Rev. 2012, 16 (7), 4406– 4414, DOI: 10.1016/j.rser.2012.04.028 [Crossref], [CAS], Google Scholar
103. Silva, R. V. S.; Pereira, V. B.; Stelzer, K. T.; Almeida, T. A.; Romeiro, G. A.; Azevedo, D. A. Comprehensive Study of the Liquid Products from Slow Pyrolysis of Crambe Seeds: Bio-oil and Organic Compounds of the Aqueous Phase. Biomass Bioenergy 2019, 123, 78– 88, DOI: 10.1016/j.biombioe.2019.02.014 [Crossref], [CAS], Google Scholar
104. Megía, P. J.; Cortese, M.; Ruocco, C.; Vizcaíno, A. J.; Calles, J. A.; Carrero, A.; Palma, V. Catalytic Behavior of Co-Based Catalysts in the Kinetic Study of Acetic Acid Steam Reforming. Ind. Eng. Chem. Res. 2020, 59 (44), 19531– 19538, DOI: 10.1021/acs.iecr.0c03599 [ACS Full Text ACS Full Text], [CAS], Google Scholar
105. Chen, J.; Sun, J.; Wang, Y. Catalysts for Steam Reforming of Bio-oil: A Review. Ind. Eng. Chem. Res. 2017, 56 (16), 4627– 4637, DOI: 10.1021/acs.iecr.7b00600 [ACS Full Text ACS Full Text], [CAS], Google Scholar
106. Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Renewable Hydrogen from Ethanol Reforming over CeO2-SiO2 Based Catalysts. Catalysts 2017, 7 (8), 226, DOI: 10.3390/catal7080226 [Crossref], [CAS], Google Scholar
107. Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Olazar, M.; Bilbao, J. Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review. Energy Fuels 2021, DOI: 10.1021/acs.energyfuels.1c01666 [ACS Full Text ACS Full Text], Google Scholar
108. Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis─A Review. Mater. Sci. Energy Technol. 2019, 2 (3), 442– 454, DOI: 10.1016/j.mset.2019.03.002 [Crossref], Google Scholar
109. Parthasarathy, P.; Narayanan, K. S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield─A Review. Renewable Energy 2014, 66, 570– 579, DOI: 10.1016/j.renene.2013.12.025 [Crossref], [CAS], Google Scholar
110. Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920, DOI: 10.1016/j.biombioe.2020.105920 [Crossref], [CAS], Google Scholar
111. Zhu, Y.; Biddy, M. J.; Jones, S. B.; Elliott, D. C.; Schmidt, A. J. Techno-Economic Analysis of Liquid Fuel Production from Woody Biomass via Hydrothermal Liquefaction (HTL) and Upgrading. Appl. Energy 2014, 129, 384– 394, DOI: 10.1016/j.apenergy.2014.03.053 [Crossref], [CAS], Google Scholar
112. Cascioli, A.; Baratieri, M. Enhanced Thermodynamic Modelling for Hydrothermal Liquefaction. Fuel 2021, 298, 120796, DOI: 10.1016/j.fuel.2021.120796 [Crossref], [CAS], Google Scholar
113. Koyuncu, I.; Yilmaz, C.; Alcin, M.; Tuna, M. Design and Implementation of Hydrogen Economy Using Artificial Neural Network on Field Programmable Gate Array. Int. J. Hydrogen Energy 2020, 45 (41), 20709– 20720, DOI: 10.1016/j.ijhydene.2020.05.181 [Crossref], [CAS], Google Scholar
114. Dawood, F.; Anda, M.; Shafiullah, G. M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45 (7), 3847– 3869, DOI: 10.1016/j.ijhydene.2019.12.059 [Crossref], [CAS], Google Scholar
115. Parra, D.; Valverde, L.; Pino, F. J.; Patel, M. K. A Review on the Role, Cost and Value of Hydrogen Energy Systems for Deep Decarbonisation. Renewable Sustainable Energy Rev. 2019, 101, 279– 294, DOI: 10.1016/j.rser.2018.11.010 [Crossref], [CAS], Google Scholar
116. Abe, J. O.; Popoola, A. P. I.; Ajenifuja, E.; Popoola, O. M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44 (29), 15072– 15086, DOI: 10.1016/j.ijhydene.2019.04.068 [Crossref], [CAS], Google Scholar
117. Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45 (38), 18753– 18771, DOI: 10.1016/j.ijhydene.2020.05.021 [Crossref], [CAS], Google Scholar
118. European Commission (EC). The European Green Deal; EC: Brussels, Belgium, 2019.Google Scholar
119. Dell, R. M.; Bridger, N. J. Hydrogen─The Ultimate Fuel. Appl. Energy 1975, 1 (4), 279– 292, DOI: 10.1016/0306-2619(75)90029-X [Crossref], [CAS], Google Scholar
120. Bockris, J. O. M. The Hydrogen Economy: Its History. Int. J. Hydrogen Energy 2013, 38 (6), 2579– 2588, DOI: 10.1016/j.ijhydene.2012.12.026 [Crossref], [CAS], Google Scholar
121. Brandon, N. P.; Kurban, Z. Clean Energy and the Hydrogen Economy. Philos. Trans. R. Soc., A 2017, 375 (2098), 20160400, DOI: 10.1098/rsta.2016.0400 [Crossref], [CAS], Google Scholar
122. European Commission (EC). A Hydrogen Strategy for a Climate Neutral Europe; EC: Brussels, Belgium, 2020.Google Scholar
123. Valente, A.; Iribarren, D.; Dufour, J. Comparative Life Cycle Sustainability Assessment of Renewable and Conventional Hydrogen. Sci. Total Environ. 2021, 756, 144132, DOI: 10.1016/j.scitotenv.2020.144132 [Crossref], [PubMed], [CAS], Google
This article is cited by 8 publications.
1. Dikun Hong, Lin Yuan, Chunbo Wang, Heyang Wang. Insight into the Competitive and Synergistic Effects during Coal/NH3 Cofiring via Reactive Molecular Dynamics Simulations. Energy & Fuels 2023, 37 (4) , 3071-3082. https://doi.org/10.1021/acs.energyfuels.2c03768
2. Fangxuan Chen, Mohamed Mehana, Hadi Nasrabadi. Molecular Simulation of Hydrogen-Shale Gas System Phase Behavior under Multiscale Conditions: A Molecular-Level Analysis of Hydrogen Storage in Shale Gas Reservoirs. Energy & Fuels 2023, 37 (3) , 2449-2456. https://doi.org/10.1021/acs.energyfuels.2c03571
3. Pooja Varma, K. Arun Joshi Reddy, D. Amaranatha Reddy, Madhusudana Gopannagari, Tae Kyu Kim. Effect of Combined Hole Storage and Blocking Interfaces on the Photoelectrochemical Water Splitting Performance of BiVO4 Photoanodes. ACS Applied Energy Materials 2022, 5 (12) , 14891-14900. https://doi.org/10.1021/acsaem.2c02362
4. Houeida Issa Hamoud, Patrick Damacet, Dong Fan, Nisrine Assaad, Oleg I. Lebedev, Anna Krystianiak, Abdelaziz Gouda, Olivier Heintz, Marco Daturi, Guillaume Maurin, Mohamad Hmadeh, Mohamad El-Roz. Selective Photocatalytic Dehydrogenation of Formic Acid by an In Situ-Restructured Copper-Postmetalated Metal–Organic Framework under Visible Light. Journal of the American Chemical Society 2022, 144 (36) , 16433-16446. https://doi.org/10.1021/jacs.2c04905
5. Daoming Chen, Zifei Xie, Yexiang Tong, Yongchao Huang. Review on BiVO4-Based Photoanodes for Photoelectrochemical Water Oxidation: The Main Influencing Factors. Energy & Fuels 2022, 36 (17) , 9932-9949. https://doi.org/10.1021/acs.energyfuels.2c02119
6. Yang Luo, Yinghong Wu, Chao Huang, Carlo Menon, Shien‐Ping Feng, Paul K. Chu. Plasma modified and tailored defective electrocatalysts for water electrolysis and hydrogen fuel cells. EcoMat 2022, 100 https://doi.org/10.1002/eom2.12197
7. Iliana D. Dimitrova, Thanos Megaritis, Lionel Christopher Ganippa, Efstathios-Al Tingas. Computational analysis of an HCCI engine fuelled with hydrogen/hydrogen peroxide blends. International Journal of Hydrogen Energy 2022, 47 (17) , 10083-10096. https://doi.org/10.1016/j.ijhydene.2022.01.093
8. Sajjad Keshipour, Alireza Asghari. A review on hydrogen generation by phthalocyanines. International Journal of Hydrogen Energy 2022, 85 https://doi.org/10.1016/j.ijhydene.2022.02.058